A mixture of mustard oil and water forms:
- a compound
- an alloy
- a homogeneous mixture
- a heterogeneous mixture
Answer
a heterogeneous mixture
Reason — Mustard oil and water are immiscible liquids, their mixtures are not uniformly distributed throughout, Hence, they form a heterogeneous mixture.
A heterogeneous mixture is:
- made up of only one kind of atoms
- made up of only one kind of molecules
- made up of different kinds of atoms and molecules.
- one that looks uniform.
Answer
made up of different kinds of atoms and molecules.
Reason — A heterogeneous mixture is made up of different kinds of atoms and molecules which do not mix uniformly.
An example of a homogeneous mixture is :
- distilled water
- tap water
- sand and water
- sawdust and water
Answer
tap water
Reason — Tap water is a homogeneous mixture because it has minerals and salts, which are evenly mixed and looks uniform throughout.
Which among the following is a set containing only mixtures ?
- gold, common salt, water, alloy
- alloy, ink, honey, ice-cream
- alloy, mercury, air, sea water
- milk, duralumin, brass, silver.
Answer
alloy, ink, honey, ice-cream
Reason — The set "alloy, ink, honey, ice-cream" has only mixtures, because,
(i) Alloy : Mixture of metals
(ii) Ink : Mixture of dyes and solvents
(iii) Honey : Mixture of sugar and water
(iv) Ice-cream : Mixture of milk, sugar, flavors and air.
Copper is not a part of the alloy :
- brass
- bronze
- steel
- duralumin
Answer
steel
Reason — Brass, bronze and duralumin has copper as their component along with other metals. Whereas, steel has iron and carbon.
Which of the following is not a mixture?
- Sugar solution
- Tap water
- Milk
- Distilled water
Answer
Distilled water
Reason — Distilled water is a pure substance, consisting of only H2O molecules without any dissolved salts, minerals, or impurities.
Which of the following is a mixture ?
- Sugar
- Honey
- Common salt
- Iron sulphide
Answer
Honey
Reason — Honey is a mixture of water and sugar.
Identify the mixtures and compounds from the following list:
Air, water, sugar, salt, milk, tea, alcohol, honey, soil, glucose.
Answer
- Mixtures — Air, milk, tea, honey, soil.
- Compounds — Water, sugar, salt, alcohol, glucose.
Select homogeneous and heterogeneous mixtures from the following:
Salt solution, petrol and water, sand and charcoal, alcohol and water, air dissolved in water, air, sea water, fruit juices, mist, brass.
Answer
Homogeneous mixture :
- Salt solution
- Alcohol and water
- Air
- Air dissolved in water
- Sea water
- Brass
- Fruit juice
Heterogeneous mixture :
- Petrol and water
- Sand and charcoal
- Mist
Select the odd one out from the following:
(a) Salt solution, sugar solution, water and alcohol, sand and water
(b) Chalk and sand, water and petrol, iron and sulphur, salt and water
(c) Gold, iron sulphide, milk, water
Answer
(a) Sand and water
Reason — Sand and water is a heterogeneous mixture, while others are homogeneous mixture.
(b) Salt and water
Reason — Salt and water is a homogeneous mixture, others form heterogeneous mixture.
(c) Milk
Reason — Milk is a mixture, whereas, iron sulphide, gold and water are pure substances.
Give two examples for each of the following types of mixtures.
(a) solid-solid
(b) solid-liquid
(c) liquid-gas
(d) gas-gas
Answer
(a) solid-solid :
- Alloys
- Sand and sugar
(b) solid-liquid :
- Sand in water
- Salt And water
(c) liquid-gas :
- Mist
- Carbon dioxide and water
(d) gas-gas :
- Air
- Helium in Oxygen
Name the components present in the following mixtures:
(a) Brass
(b) Duralumin
(c) Tap water
(d) Bronze
Answer
(a) Brass — Copper and zinc.
(b) Duralumin — Aluminium, copper, magnesium and manganese.
(c) Tap water — Water, dissolved salts, dissolved gases, minerals
(d) Bronze — Copper, Tin and Zinc.
Define the following with an example for each:
(a) Pure substance
(b) Impure substance
(c) Alloy
(d) Solution
(e) Heterogeneous mixture
(f) Homogeneous mixture
Answer
(a) Pure substances are either elements or compounds. They are homogeneous substances that contain the same kind of atoms or molecules and have a definite set of physical and chemical properties.
(b) A substance in which some other substances are also present in smaller or larger amounts is called a mixture or impure substance.
(c) A homogeneous (solid) mixture of two or more metals or a metal and a non-metal is called an alloy.
Example :
- Brass: Copper + Zinc
- Bronze: Copper + Tin + Zinc
(d) The homogeneous mixture of water (or any other solvent) and a substance soluble in it (solute) is called a solution.
(e) A mixture in which its constituents are not uniformly distributed throughout its volume and can easily be seen separately is called a heterogeneous mixture.
(f) A mixture in which its constituents are uniformly distributed throughout its volume and cannot be seen separately is called a homogeneous mixture.
Give one difference between pure substances and mixtures.
Answer
The difference between pure substances and mixture are given below:
| Pure substance | Mixture |
|---|---|
| They contain same kind of atoms or molecules. | They contain different kind of atoms or molecules. |
| Example : Gold, sugar, etc. | Example : Air, honey, etc. |
What do you observe when a magnet is brought near a mixture of iron and sulphur ?
Answer
When a magnet is brought near a mixture of iron and sulphur, the iron fillings being magnetic in nature are attracted by the magnet from the mixture.
Give reason:
Why do sugar and water retain their individual properties in a sugar solution?
Answer
A mixture has no definite set of properties. For example, a mixture of sugar and water will have different physical and chemical properties if they are mixed in varying proportions. Their densities, boiling points, etc., depend upon the amounts of the two components present in it.
Give reason:
Why do petrol and water form a heterogeneous mixture ?
Answer
Petrol and water form a heterogeneous mixture because they are immiscible liquids, they do not mix uniformly due to differences in their physical and chemical properties. Petrol floats on top of water, because the density of petrol is less than water.
Give reason:
Why does sulphur dissolve when carbon disulphide is added to a mixture of iron and sulphur but not when it is added to iron sulphide ?
Answer
Iron is grey coloured and sulphur is a yellow substance. When we form a mixture of iron and sulphur, they retain their individual property, but when you heat this mixture, it will form a compound called as iron sulphide.
So, when we dissolve the mixture of iron and sulphur in Carbon disulphide, sulphur dissolves to form yellow coloured solution leaving behind iron at the bottom. In case of iron sulphide compound, it will not dissolve and settles down at the bottom.
State: Four differences between compounds and mixtures.
Answer
The differences between compound and mixtures are given below:
| Compound | Mixture |
|---|---|
| A compound is a pure substance. | A mixture is an impure substance. |
| Compounds are always homogeneous. | Mixtures may be homogeneous or heterogeneous. |
| Properties of components are changed during the formation of a compound. | Mixtures retain the properties of its components. |
| Compounds have fixed melting points and boiling points. | Mixtures do not have fixed melting points and boiling points. |
| Components of compounds can be separated only by chemical methods. | Components of mixtures can be separated by simple physical methods. |
State: Three differences between water and air.
Answer
The difference between water and air are given below;
| Water | Air |
|---|---|
| Water is a compound. | Air is a mixture. |
| Water is made up of hydrogen and oxygen in a fixed proportion by mass. | Air contains oxygen, nitrogen, carbon dioxide and water vapour, but they are not in a fixed proportion. Their amount varies from place to place. |
| The properties of water are completely different from those of its elements. | Air retains the properties of its component gases. |
| Water is represented by a molecular formula H2O. | Air cannot be represented by any formula. |
(a) Define 'mixture'.
(b) List four characteristics of a mixture.
Answer
(a) Mixtures can be defined as impure substances which are formed by mixing two or more pure substances (elements and/or compounds) in any proportion such that, they do not undergo any chemical change and retain their individual properties.
(b) Four characteristics of a mixture :
- A mixture consists of two or more substances mixed together without any chemical force acting on or between them.
- Mixtures do not have any fixed composition, i.e. they can have their components in varying proportions.
- In mixtures, components are loosely held together and they retain their individual properties.
- A mixture has no definite set of properties.
The process of adding a chemical substance to help the suspended solid particles to deposit as sediment faster is called:
- loading
- sedimentation
- decantation
- filtration
Answer
loading
Reason — The process of helping fine solid particles in a solid-liquid mixture to settle faster, by adding a special chemical, is called loading or coagulation.
Salt is separated from sea water by:
- evaporation
- sublimation
- magnetic separation
- filtration
Answer
evaporation
Reason — Salt from sea water is separated by evaporation. Sea water is collected in shallow beds and allowed to evaporate in the sun. When all the water is evaporated, salt is left behind.
A gas dissolved in a liquid can be separated by:
- filtration
- boiling
- using magnet
- sublimation
Answer
boiling
Reason — A mixture of gas in liquid can be separated by heating or boiling. Dissolved gas escapes from the liquid on heating or boiling.
Camphor can be separated from a mixture of sand and camphor by :
- filtration
- evaporation
- sublimation
- winnowing
Answer
sublimation
Reason — Camphor can be separated from a mixture of sand and camphor by sublimation. The camphor sublimes and escapes as vapour, while the sand is left behind.
A separating funnel is used to separate :
- miscible liquids
- sugar solution
- chalk powder and water
- immiscible liquids
Answer
immiscible liquids
Reason — A separating funnel is a laboratory tool used to separate immiscible liquids. The denser liquid settles at the bottom, and the lighter one floats on top. When we open the stopcock the bottom layer drains first, effectively separating the two.
Fill in the blanks :
(a) The substances that make a mixture are called its ............... or ............... .
(b) ............... is a process to separate solids dissolved in liquids.
(c) Mist is a ............... mixture of droplets of water and air.
(d) Clay is separated from water by the method of ............... .
(e) When cereals are washed before cooking, water is separated from the cereals by ............... .
(f) ............... is a process to obtain a very pure form of a solid dissolved in a liquid.
(g) Ammonium chloride can be separated from common salt by the method of ............... .
(h) The solid particles which remain on the filter paper are called ............... and the liquid which passes through it is called ............... .
(i) The process of transferring the clear liquid above the solid particles which settle at the bottom of the container is known as ............... .
(j) ............... is a method used for the separation of an insoluble solid from a solid-liquid mixture.
Answer
(a) The substances that make a mixture are called its components or constituents.
(b) Evaporation is a process to separate solids dissolved in liquids.
(c) Mist is a heterogeneous mixture of droplets of water and air.
(d) Clay is separated from water by the method of filtration.
(e) When cereals are washed before cooking, water is separated from the cereals by decantation.
(f) Evaporation is a process to obtain a very pure form of a solid dissolved in a liquid.
(g) Ammonium chloride can be separated from common salt by the method of sublimation.
(h) The solid particles which remain on the filter paper are called residue and the liquid which passes through it is called filtrate.
(i) The process of transferring the clear liquid above the solid particles which settle at the bottom of the container is known as decantation.
(j) Filtration is a method used for the separation of an insoluble solid from a solid-liquid mixture.
Write "true" or "false" for the following statements:
(a) A pure substance consists of only one kind of atom or molecule. ...............
(b) The components of a mixture are present in a fixed ratio. ...............
(c) Common salt is separated from its solution in water by decantation. ...............
(d) Winnowing is a process to remove small stones from grains. ...............
(e) Gold jewellery is a homogeneous mixture of metals. ...............
(f) Air can be separated from water by filtration. ...............
(g) Salt and air dissolved in water add taste to water. ...............
(h) Steel is an alloy of iron and aluminium. ...............
Answer
(a) True
(b) False
Correct Statement — A mixture has no fixed composition, i.e. it is formed by mixing two or more substances in any ratio without any chemical reaction.
(c) False
Correct Statement — Common salt is separated from its solution in water by evaporation
(d) False
Correct Statement — Winnowing removes lighter husk from heavier grains, whereas, small stones are removed by hand picking or sieving.
(e) True
(f) False
Correct Statement — Air can be separated from water by heating or boiling.
(g) True
(h) False
Correct Statement — Steel is an alloy of iron and carbon.
Match the following:
| Column A | Column B |
|---|---|
| (a) Heterogeneous mixture | (i) Homogeneous mixture |
| (b) Evaporation | (ii) Rice from husk |
| (c) Sublimation | (iii) Iron and sulphur |
| (d) Compounds | (iv) Sea Water |
| (e) Winnowing | (v) Ammonium chloride and salt |
Answer
| Column A | Column B |
|---|---|
| (a) Heterogeneous mixture | (iii) Iron and sulphur |
| (b) Evaporation | (iv) Sea Water |
| (c) Sublimation | (v) Ammonium chloride and salt |
| (d) Compounds | (i) Homogeneous mixture |
| (e) Winnowing | (ii) Rice from husk |
Give one word for the following:
(a) The solid which is left on the filter paper after filtration.
(b) The solid particles which separate out from the solution on slow evaporation.
(c) The solid particles that settle at the bottom of the beaker in a heterogeneous mixture of a solid and a liquid.
(d) The clear liquid which is poured out after sedimentation.
(e) The technique used to separate the light particles from heavy particles using the flow of wind.
Answer
(a) Residue
(b) Crystals
(c) Sediment
(d) Supernatant liquid
(e) Winnowing
Name the process by which the components of the following mixtures can be separated.
(a) Powdered glass and sugar
(b) Chalk powder and iron filings
(c) Chaff and grain
(d) Salt and water
(e) Wheat and sugar
(f) Sand and camphor
(g) Sugar and water
Answer
(a) Solvent extraction method and filtration
(b) Magnetic separation
(c) Winnowing
(d) Evaporation
(e) Sieving
(f) Sublimation
(g) Evaporation
Rearrange the following to get meaningful words:
(all are methods to separate the components of mixtures).
(a) OIRATVOPEAN
(b) DNOITITLLIAS
(c) FTOLINTRAI
(d) SINIVEG
Answer
(a) EVAPORATION
(b) DISTILLATION
(c) FILTRATION
(d) SIEVING
Name:
(a) two substances which can sublime
(b) two substances soluble in water
(c) two substances insoluble in water
(d) four substances that can be used as filters.
Answer
(a) Two substances which can sublime :
- Camphor
- Naphthalene
(b) Two substances soluble in water :
- Sugar
- Salt
(c) Two substances insoluble in water :
- Sand
- Sawdust
(d) Four substances used as filters :
- Layer of sand
- Charcoal
- Cotton
- Filter paper
Give one example for each of the following types of mixtures.
(a) Solid-solid heterogeneous mixture
(b) Solid-liquid heterogeneous mixture
(c) Solid-liquid homogeneous mixture
Answer
(a) Rice and husk
(b) Chalk powder and water
(c) Salt and sea water
Define:
(a) Filtration
(b) Sublimation
(c) Evaporation
(d) Sedimentation
(e) Decantation
(f) Distillation
Answer
(a) The process of separating insoluble solid particles from a liquid by allowing it to pass through a filter is called filtration.
(b) The process in which a solid changes directly into its vapour on heating without changing into its liquid state is called sublimation.
(c) Evaporation is the process of converting a liquid into its vapour state, either by exposing it to air or by heating.
(d) The settling down of suspended, insoluble, heavy solid particles in a solid-liquid mixture when left undisturbed is called sedimentation.
(e) The process of pouring out the clear liquid, without disturbing the sediment, is called decantation.
(f) Distillation is the method of getting a pure liquid from a solution by evaporating and then condensing the vapours.
Why do we need pure substances ?
Answer
We need pure substances because of the following reasons :
- Impurities in food, water, or cosmetics can cause allergic reactions, poisoning, or contamination.
- Pure substances have fixed boiling and melting points, making them reliable for calibration and standards. Whereas, mixtures often have variable physical properties.
- Pure substances have a known chemical composition, which is critical when calculating concentrations, dosages, or reactions.
- In industries like pharmaceuticals, electronics, and food production, impurities can ruin a product or make it unsafe.
Give reason:
Sand and sawdust cannot be separated by hand picking.
Answer
Handpicking is used when the undesirable particles to be separated should be large enough in size, or may be coloured. But, in case of mixture of sand and sawdust, both the substance are of almost same colour and sawdust has very fine particles, so it is hard to separate them by hand picking.
Give reason:
Magnet is used to separate a mixture of iron and sulphur.
Answer
In magnetic separation, a magnet is moved over the mixture of iron and sulphur, iron being magnetic in nature gets attached to the magnet and is separated.
Give reason:
Alum is used in purification of river water.
Answer
Water from a river contains very fine clay particles. To make them settle at a faster rate, a chemical substance called alum in powdered form is added to such mixtures. It dissolves in water and forms clusters with clay and dust particles making them heavier and, increasing the rate of sedimentation.
The following diagram shows a method employed for the separation of a mixture. Study the same and answer the following questions:
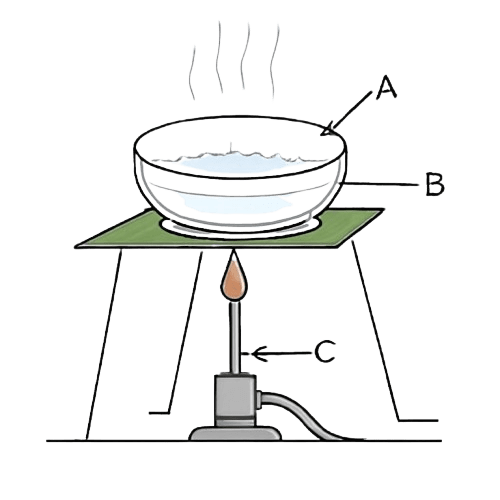
(a) Label the parts shown as A, B and C.
(b) Write the name of the method depicted above.
(c) What kind of mixture is separated by this method?
(d) How can this process be made faster ?
(e) What is one disadvantage of this process ?
Answer
(a) The parts shown as A, B, and C indicate the following:
A → Liquid
B → Evaporating dish
C → Bunsen burner
(b) Evaporation is depicted in the given picture.
(c) Homogeneous solid-liquid mixture is separated by this method.
(d) Evaporation can be made faster by heating.
(e) In evaporation process, the solvent is lost to the environment in vapour form.