The diameter of a molecule is approximately :
- 1 cm
- 10 cm
- 10-10 m
- 1 m
Answer
10-10 m
Reason — A molecule is very small in size which is about 10-10 m to 10-9 m.
The inter-molecular forces are strongest in :
- solids
- liquids
- gases
- both (i) and (ii)
Answer
solids
Reason — The inter-molecular force of attraction between the particles is more in solids, less in liquids and least in gases.
The molecules :
- in solid, liquid and gas, move freely anywhere.
- in a solid, move freely within its boundary.
- in a liquid, move within its boundary.
- in a gas, move only within its boundary.
Answer
in a liquid, move within its boundary.
Reason — The molecules in a solid are fixed at their positions and can only vibrate about their mean positions while in a liquid they can move within the boundary and moves freely in space in gas.
Solids are :
- more dense
- less dense
- least dense
- highly compressible
Answer
more dense
Reason — Solids have strong force of attraction (inter-molecular force) and least inter-molecular spaces between it's molecules which makes them more closely packed state and thus are more dense than liquids and gases.
The inter-molecular forces in liquids are :
- as strong as in solids
- stronger than in solids
- weaker than in solids
- weaker than in gases
Answer
weaker than in solids
Reason —
Solids : Molecules are tightly packed, and intermolecular forces are strongest, giving them a fixed shape and volume.
Liquids : Molecules are less tightly packed compared to solids, so the intermolecular forces are weaker — allowing them to flow but still maintain a definite volume.
Gases : Molecules are far apart, and intermolecular forces are negligible — they can expand to fill any container.
The diagram below shows the arrangement of molecules in three states of matter.
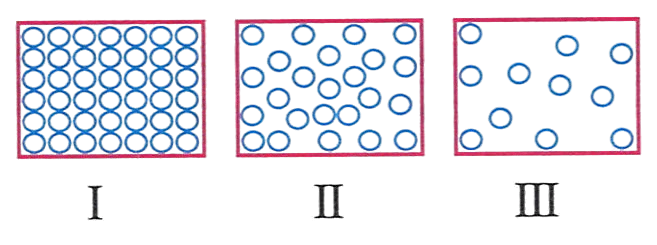
The arrangement of molecules in drinking water would look like.
- I
- II
- III
- all of these
Answer
II
Reason — As drinking water belongs to liquid state which is less closely packed than solid and more packed than gas.
Fill in the blanks :
(a) All the molecules of a substance are ............... .
(b) The inter-molecular spacing is ............... in solids ............... in liquids and ............... in gases.
(c) The molecular motion in liquid and gas is in ............... path.
(d) In a solid, the molecules ............... but they remain at their fixed positions.
(e) The inter-molecular forces are the weakest in ............... .
(f) A solid exerts pressure ............... .
(g) Gases are ............... dense.
(h) Solids are ............... rigid.
Answer
(a) All the molecules of a substance are identical.
(b) The inter-molecular spacing is least in solids more in liquids and still more in gases.
(c) The molecular motion in liquid and gas is in zig-zag path.
(d) In a solid, the molecules vibrate on either side but they remain at their fixed positions.
(e) The inter-molecular forces are the weakest in gases.
(f) A solid exerts pressure downwards on its base.
(g) Gases are least dense.
(h) Solids are most rigid.
Write true or false for each statement :
(a) The molecules of each substance are identical.
(b) The inter-molecular forces are effective at all distances between the two molecules.
(c) The molecules in a substance are in random motion.
(d) In a gas, the molecules can move anywhere in space.
(e) Liquids are less viscous than gases.
Answer
(a) False because different substances (e.g. air, water etc.) contain different types of molecules, so they are not identical
(b) False as intermolecular forces act only at very short distances (a few nanometers). At large distances, they become negligible.
(c) True since the particles of matter are not at rest, but they move randomly in all possible directions in a zig zag path.
(d) True as the inter-molecular forces are very weak in a gas which makes the molecules wide apart and hence, they can move freely in space.
(e) False because liquids are generally more viscous than gases because their molecules are closer together, leading to more friction between different layers.
Match the following columns :
| Column A | Column B | ||
|---|---|---|---|
| (a) | A molecule is composed of | (i) | does not exist free in nature. |
| (b) | Ice, water and water vapour | (ii) | can vibrate only up to about 10-10 m from their mean positions. |
| (c) | An atom | (iii) | atoms. |
| (d) | Gases | (iv) | are the three states of water. |
| (e) | The molecules of a solid | (v) | occupy space |
Answer
| Column A | Column B | ||
|---|---|---|---|
| (a) | A molecule is composed of | (iii) | atoms. |
| (b) | Ice, water and water vapour | (iv) | are the three states of water. |
| (c) | An atom | (i) | does not exist free in nature. |
| (d) | Gases | (v) | occupy space |
| (e) | The molecules of a solid | (ii) | can vibrate only up to about 10-10 m from their mean positions. |
Define matter. What is its composition?
Answer
Matter is defined as any substance that occupies space and has mass. It is made up of molecules which are very small in size (~ 10-9 m).
Name the three states of matter.
Answer
Three states of matter are :
- Solid
- Liquid
- Gas
What is a molecule?
Answer
A molecule is the smallest particle of a substance that is made up of one or more than one atoms of the same kind or of different kinds.
Mention one example each of a monoatomic and a diatomic molecule.
Answer
Monoatomic Molecule : Neon (Ne)
Diatomic Molecule : Hydrogen Molecule (H2)
What do you mean by inter-molecular spacing?
Answer
The spacing between molecules of matter is called inter-molecular space.
What do you mean by inter-molecular forces?
Answer
The force of attraction between the constituent particles of matter is called intermolecular force of attraction.
What are the forces of cohesion and adhesion ?
Answer
Force of cohesion (Cohesive Force) : The force of attraction between the particles of same substances is called the force of cohesion or cohesive force.
Force of adhesion (Adhesive Force) : The force of attraction between the particles of two different substances is called the force of adhesion or adhesive force.
State three characteristics of molecules of matter which determine its solid, liquid and gaseous state.
Answer
The three characteristics which decide the state of a substance as a solid, liquid or gas are :
- Inter-molecular space
- Force of attraction between the molecules
- Movement of molecules
The molecules in a substance are in motion. What type of path do they follow?
Answer
The molecules in a substance follow zig zag path when they are in motion.
Complete the following :
(a) Solid Liquid
(b) ............... Gas
Answer
(a) Solid Liquid
(b) Liquid Gas
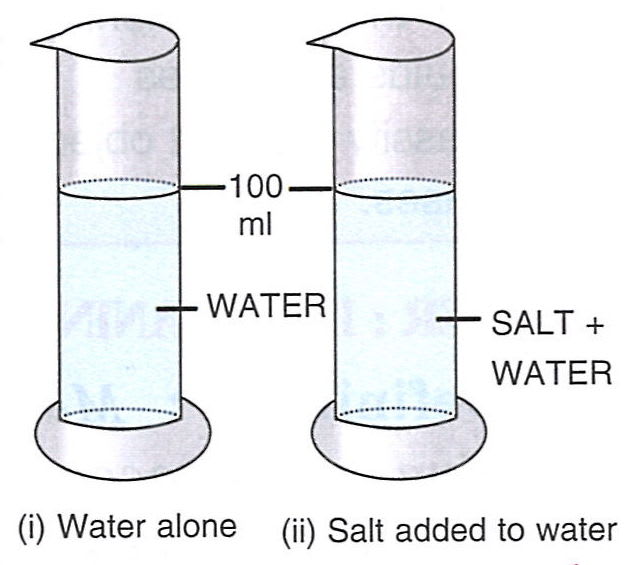
Describe a simple experiment to illustrate the existence of inter-molecular spacing.
Answer
Experiment :
Take 100 ml of water in a measuring cylinder. Add 20 gram of salt in water gently and stir it well so as to dissolve the salt well in water. It is noticed that the level of water does not change as it can be seen from below figure. It shows that the particles of salt have occupied the spaces between the particles of water.
How do solids, liquids and gases differ in their following properties :
(a) Size
(b) Shape
(c) Density?
Answer
| Properties | Solid | Liquid | Gas | |
|---|---|---|---|---|
| (a) | Size (volume) | Definite | Definite | Indefinite, acquires the size available |
| (b) | Shape | Definite | Indefinite, acquires the shape of the container | Indefinite, acquires the shape of the container |
| (c) | Density | Highly dense | Less dense | Least dense |
Describe a simple experiment to illustrate that molecules are not at rest, but they constantly move.
Answer
The following experiment can be performed to illustrate that molecules are not at rest, but they constantly move :
Take a beaker. Fill it partly with water. Add some lycopodium powder in the beaker containing water. Stir the contents of the beaker with a glass rod. Take out few drops of this suspension on a glass plate. Place the plate on the table and illuminate it with a table lamp. Observe the glass plate through a microscope. It is found that the fine particles of lycopodium powder move rapidly in a random manner and their path is zig zag as shown in figure below.

The reason is that the particles of water are in random motion which collide with the suspended fine particles of lycopodium powder and make them move in a zig zag path.
Write down five general properties of solids, liquids and gases.
Answer
Five general properties of solids, liquids and gases are given below:
| S. No. | Solid | Liquid | Gas |
|---|---|---|---|
| 1. | A solid has a definite shape and a definite size (i.e., length, area and volume) | A liquid has a definite volume, but not a definite shape. | A gas has neither a definite volume nor a definite shape. |
| 2. | The molecules in a solid are closely packed. | The molecules in a liquid are loosely packed. | The molecules in a gas are wide apart. |
| 3. | The molecules in a solid are fixed at their positions. They can only vibrate about their mean positions. | The molecules in a liquid can move within the boundary of the liquid. | The molecules of a gas can move freely in space. |
| 4. | The inter-molecular forces are very strong. | The inter-molecular forces are less strong (moderate). | The inter-molecular forces are weak. |
| 5. | Solids are highly rigid. | Liquids are less rigid. | Gases are non-rigid. |
Give the molecular model for a solid and use it to explain why a solid has a definite volume and a definite shape.
Answer
Features of molecular model for a solid are :
- There is a strong force of attraction (strong inter-molecular force) between the molecules of a solid.
- The molecules in a solid are closely packed with negligible intermolecular space, so solids cannot be compressed much. Their orderly arrangement gives them a definite shape.
- The molecules vibrate on either side of their mean positions but they do not leave their positions. Therefore solids have a definite size.
- The molecules of a solid are packed tightly and so they generally have a high density.
Thus, this model concludes that solids are rigid, they have a definite size, definite shape and a definite volume.
Describe the molecular model for a liquid. How does it explain that a liquid has no definite shape, but has a definite volume ?
Answer
Features of molecular model for a liquid are :
- The molecules in a liquid are loosely packed due to weaker attractive forces than in solids. They can move over each other, within the boundary of the liquid, so a liquid has a definite volume but no definite shape.
- The inter-molecular space in a liquid is greater than that in a solid, so they generally have low density as compared to a solid, i.e., they are more compressible.
- The motion of molecules in a liquid is irregular and random within the boundary of the liquid.
Thus, this model concludes that liquids do not have a definite shape, but have a definite volume and can flow from a higher to a lower level. They show the property of viscosity and surface tension because of the cohesive forces.
A gas has neither a definite volume nor a definite shape. Describe the molecular model to explain it.
Answer
Features of molecular model for a gas are :
- The molecules of a gas lie much farther apart than they lie in a liquid or a solid. Thus, the density of gases is very low.
- There is negligible force of attraction between the molecules of a gas, so they are free to move in the entire space available to them.
- The molecules of a gas move much faster than they move in liquids, and therefore they are infact in a state of incessant random motion, moving in all possible directions at all possible speeds.
- The molecules of a gas are far apart and there is enough space available for compression. Thus, gases can easily be compressed.
- During motion, the molecules of a gas collide with one another and also with the wall of the vessel. In each collision, the direction of motion of the molecule changes, so the momentum changes.
- A gas exerts pressure on the wall of its container due to the continuous collisions of its molecules with the wall.
Thus, this model concludes that a gas has neither a definite shape nor a definite volume, but it can flow and is easily compressible.
Distinguish between the three states of matter — solid, liquid and gas on the basis of their molecular models.
Answer
| S. No. | Properties | Solid | Liquid | Gas |
|---|---|---|---|---|
| 1. | Mass | Definite | Definite | Definite |
| 2. | Shape | Definite | Acquires the shape of the container | Acquires the shape of the container |
| 3. | Volume | Definite | Definite | Indefinite, acquires the volume available |
| 4. | Compressibility | Not compressible | Negligibly compressible | Highly compressible |
| 5. | Fluidity | Not possible | Can flow | Can flow |
| 6. | Rigidity | Highly rigid | Less rigid | Not rigid |
| 7. | Diffusion | Slow | Fast | Very fast |
| 8. | Number of free surfaces | Any number of free surfaces | Only one free surface | None |
| 9. | Packing of molecules | Very closely packed | Less closely packed | Least closely packed |
| 10. | Inter-molecular forces | Strongest | Slightly weaker than in solids | Negligible |
| 11. | Expansion on heating | Low | More than solids | More than liquids |
| 12. | Motion of constituent molecules | Only vibrate on either side of their mean positions | Move in all directions but within the boundary of the liquid | Move in a random manner in all space available |
| 13. | Pressure | Only at base downwards | At all points in all directions inside the boundary of the liquid | On the walls of the container |
| 14. | Viscosity | No | More viscous | Least viscous |
| 15. | Surface Tension | No | Due to cohesive force tends to occupy minimum surface area | No |
Distinguish between solids, liquids and gases on the basis of their following properties:
(a) compressibility
(b) fluidity
(c) rigidity
(d) expansion on heating
Answer
| Properties | Solid | Liquid | Gas | |
|---|---|---|---|---|
| (a) | Compressibility | Not compressible | Negligibly compressible | Highly compressible |
| (b) | Fluidity | Not possible | Can flow | Can flow |
| (c) | Rigidity | Highly rigid | Less rigid | Not rigid |
| (d) | Expansion on heating | Low | More than solids | More than liquids |
What do you mean by change of state of matter? Explain :
(a) the change of a solid into a liquid at a constant temperature, and
(b) the change of a liquid into a gas at a constant temperature.
Answer
A change of state of matter refers to the physical transformation of a substance from one state (solid, liquid, or gas) to another due to a change in temperature.
(a) The process of changing of a substance from the solid state into its liquid state on absorption of heat at a particular temperature, called the melting point, is called melting in which the heat energy absorbed by the substance increases the amplitude of vibrations of the molecules of the solid and a stage is reached at the melting point when the molecules acquire sufficient energy to overcome the force of attraction between them and they become free to move. The solid thus changes into a liquid.
Solid Liquid
(b) The process of change of a substance from the liquid state to its gaseous state at a particular temperature, called the boiling point, is called boiling in which the heat energy absorbed by a substance in liquid state increases the energy of its molecules due to which they begin to move rapidly. Thus a liquid changes into a gas.
Liquid Gas
Given below is a crossword puzzle based on this lesson. Read the clues across and clues downwards and fill up the blank squares.

Across :
- Matter is made up of ............... .
- The ............... forces are less strong in liquids.
- A solid has ............... shape.
Down :
- A solid cannot be ............... .
- Liquids are ............... rigid.
- Gases are ............... compressible.
Answer
- Matter is made up of atoms .
- A solid cannot be compressed .
- The intermolecular forces are less strong in liquids.
- Liquids are less rigid.
- A solid has definite shape.
- Gases are highly compressible.