Water exists in all the three states. Discuss.
Answer
Water exists in all the three physical states : as solid (ice), as liquid (water) and as gas (water vapour). It occurs in both free as well as combined states.
Occurrence of water in free state:
- Solid state — In the form of ice, snow, frost.
- Liquid State —
- On the earth's surface as river, water, lake water, sea water, spring water.
- Below the earth's surface in well water and moisture accumulation in the soil.
- Above the earth's crust as dew.
- Gaseous state [vapour] — as water vapour, clouds, mist, fog.
Occurrence of water in combined state :
- Water occurs in the combined form in all living matter i.e., plants and animals.
- Water is present in hydrated salts e.g., MgCl2.6H2O and in certain minerals.
- Earth's surface - Covers nearly 75% of earth's surface.
- Human body - Nearly, 70% of the body weight.
- Food products - Green vegetables [80-90%], Milk [80-85%], Dry cereals [3-5%]
Why is water considered a compound.
Answer
Henry Cavendish synthesized water from it's elements [2 vols. of hydrogen and 1 vol. of oxygen] by igniting the elements in their respective ratio, thereby leading to the conclusion that water is not an element but a compound of hydrogen and oxygen combined in the ration 2:1.
(a) Why does temperature in Mumbai and Chennai not fall as low as it does in Delhi.
(b) Give the properties of water responsible for controlling the temperature of our body.
Answer
(a) Mumbai and Chennai are on the shores of the sea. As water has high specific heat capacity, the presence of a large amount of water is able to modify the climate of the nearby land areas, making them warmer in winter and cooler in summer. Land and sea breeze also take place because of this great moderating property. Hence, the temperature in Mumbai and Chennai does not fall as low as it does in Delhi.
(b) Properties of water responsible for controlling the temperature of our body:
- High specific heat capacity — Water has a high specific heat capacity enabling it to absorb heat from the body and release it to the environment without causing significant changes in its own temperature.
- High latent heat of vaporization — As water has a high latent heat of vaporization, it requires a large amount of heat energy to change from liquid to gas. This property allows sweat to evaporate from the skin, taking heat from the body and cooling it down.
'Water is a universal solvent'. Comment.
Answer
Water dissolves many substances, forming aqueous solutions (water solutions). Not only solids but gases and other liquids can also dissolve in water to a large extent. For the same reason, water is called a universal solvent.
What causes the violence associated with torrential rain?
Answer
The sudden release of latent heat of condensation causes the violence associated with torrential rain.
Which property of water enables it to modify the climate ?
Answer
Due to it's high specific heat capacity, the presence of a large amount of water is able to modify the climate of the nearby land areas, making them warmer in winter and cooler in summer. Land and sea breezes also take place because of this great moderating property of water.
Density of water varies with temperature. What are its consequences?
Answer
At 4°C, water has its maximum density, 1g/cm3 or 1000 kg/m3, and minimum volume. Its density decreases as the temperature increases or decreases from this point. The consequences of this are:
- Water expands on freezing, i.e., 92 volumes of water become 100 volumes of ice. Therefore, with relative density of ice being 0.92, it floats on water.
- It enables marine life to exist in the colder regions of the world because even when the water freezes on the surface, it remains in liquid state below the ice layer as the density of water is greater than the density of ice and ice is a bad conductor of heat.
- It can cause pipes to burst in winter season because when water freezes it expands slightly.
What is the effect of impurities present in water on melting point and boiling point of water?
Answer
The boiling point of water increases and the melting point of water decreases due to the presence of dissolved impurities in it.
How do fishes and aquatic animals survive in winters when the pond gets covered with thick ice?
Answer
The property of anomalous expansion water enables marine life to exist in the colder regions of the world because even when the water freezes on the surface, it remains in liquid state below the ice layer as the density of water is greater than the density of ice and ice is a bad conductor of heat.

The properties of water are different from the properties of the elements of which it is formed. Discuss.
Answer
Water is composed of Hydrogen and Oxygen in the ratio of 2:1. Its properties are distinct from those of its component elements in the following ways:
| Properties | Hydrogen | Oxygen | Water |
|---|---|---|---|
| State at room temperature | Gas | Gas | Liquid |
| Boiling Point | -253°C | -183°C | 100°C |
| Melting point | –259°C | -218.8°C | 0°C |
| Flammability | Explosive | Necessary for combustion | Extinguishes flame |
| Polarity | Non-polar | Non-polar | Polar |
How is aquatic life benefitted by the fact that water has maximum density at 4°C?
Answer
At 4°C, water has its maximum density, 1g/cm3 or 1000 kg/m3, and minimum volume. Its density decreases as the temperature increases or decreases from this point. This enables marine life to exist in the colder regions of the world because even when the water freezes on the surface, it remains in liquid state below the ice layer as the density of water is greater than the density of ice and ice is a bad conductor of heat.

What are your observations and conclusion when tap water is boiled and evaporated in watch glass?
Answer
Observation — On looking at the watch glass against light, a number of concentric rings of solid matter are seen. These are the dissolved solids left behind after evaporation of water.
Conclusion — Tap water contains dissolved solids.
What is the importance of dissolved salts in water?
Answer
Importance of dissolved salts in water are as follows:
- Salts and minerals are essential for the growth and development of plants.
- They add taste to water.
- They supply the essential minerals needed by our bodies.
State the importance of the solubility of CO2, and O2 in water.
Answer
Solubility of CO2, and O2 in water is important for the following reasons:
- Marine life like fish use the oxygen of the air dissolved in water for respiration and thus aquatic life is sustained. 1 dm3 (1 litre) of water contains 40 cm3 of dissolved oxygen.
- Aquatic plants make use of dissolved carbon dioxide for photosynthesis, i.e., to prepare their food.
6CO2 + 12H2O C6H12O6 + 6O2 + 6H2O - Carbon dioxide dissolved in water reacts with limestone to form calcium bicarbonate.
CaCO3 + CO2 + H2O ⟶ Ca(HCO3)2
Marine organisms such as snails, oysters, etc., extract calcium carbonate from calcium bicarbonate to build their shells.
How is air dissolved in water different from ordinary air?
Answer
Ordinary air contains 78% nitrogen, 21% oxygen and 0.01% carbon dioxide. But oxygen is more soluble in water as compared to nitrogen. Hence, the composition of air dissolved is different from ordinary air.
The composition of air dissolved in water is 33% oxygen, 66% nitrogen and 1% carbon dioxide.
Identify A, B, C and D; first one is done for you.

Answer
(B) Latent heat of vaporization — It is the heat energy required to change water into its vapour at its boiling point without any change in temperature.
(C) Latent heat of condensation — It is the heat energy released when a gas converts to liquid.
(D) Latent heat of solidification — It is the heat energy released when a liquid converts to solid.
Explain why:
(a) Boiled or distilled water tastes flat.
(b) Ice at zero degree centigrade has greater cooling effect than water at 0°C.
(c) Burns caused by steam are more severe than burns caused by boiling water.
(d) Rivers and lakes do not freeze easily?
(e) Air dissolved in water contains a higher proportion of oxygen.
(f) If distilled water is kept in a sealed bottle for a long time, it leaves etchings on the surface of the glass.
(g) Rain water does not leave behind concentric rings when boiled.
Answer
(a) Pure water is tasteless. The taste in water is due to the gases and solids dissolved in it i.e., impurities present in it. As boiled and distilled water are pure containing no impurities hence they taste flat i.e., are tasteless.
(b) The latent heat of fusion of ice is 336 J g-1 so it absorbs 336 J of heat and changes to water at 0°C and then water at 0°C absorbs heat and temperature is raised. Therefore, ice at 0°C absorbs extra heat in comparison to water at 0°C (due to latent heat of fusion). Hence, ice at 0°C has greater cooling effect than water at 0°C.
(c) Steam has a higher heat content on account of high specific latent heat of condensation that is 2268 J g-1. Hence, steam at 100°C carries more heat than water. Therefore, burns caused by steam are more severe than burns caused by boiling water.
(d) Rivers and lakes do not freeze easily because the specific latent heat of fusion of ice is sufficiently high (= 336 J g-1). The water in lakes and rivers will have to liberate a large quantity of heat to the surrounding before freezing. The layer of ice formed over the water surface, being a poor conductor of heat, will also prevent the loss of heat from the water of lake, hence the water does not freeze all at once.
(e) Air dissolved in water contains a higher proportion of oxygen because oxygen is more soluble in water compared to nitrogen. The composition of air dissolved in water is 33% oxygen compared to 21% in ordinary air.
(f) Substances that are apparently insoluble in water actually dissolve in it in traces. Even, when we put water in a glass vessel, an extremely small amount of glass dissolves in it. It is for this reason that when distilled water is kept in a sealed bottle for a long time, it leaves etchings on the inside surface of the glass.
(g) As rainwater does not contain dissolved solids hence, it does not leave behind concentric rings when boiled.
To make a saturated solution, 136 g of a salt is dissolved in 500 g of water at 293 K. Find its solubility at this temperature.
Answer
Given,
Mass of solute = 136 g
Mass of solvent = 500 g
At the temperature 293k
Solubility = x 100
= x 100
= 27.2 g
∴ The solubility of given salt at 293k is 27.2 g
A solution contains 15 g of sodium chloride in 285 g of water. Calculate the concentration of the solution.
Answer
Given,
Mass of solute = 15 g
Mass of solvent = 285 g
Mass of solution = Mass of solute + Mass of solvent
= 15 + 285
= 300
Mass percent = x 100
= x 100
= 5%
∴ Concentration of sodium chloride in 285 g of water is 5%
4 litres of an organic compound, acetone, is present in 90 litres of an aqueous solution. Calculate its volume percent.
Answer
Given,
Volume of solute = 4 litres
Volume of solution = 90 litres
Volume percent = x 100
= x 100
= 4.44 %
∴ Volume percent of acetone in 90 litres of aqueous solution is 4.44 %
The following table gives the solubility of different salts at different temperatures.
| Temperature (in K) | Substance dissolved (in g) | |||
|---|---|---|---|---|
| KNO3 | NaCl | KCl | NH4Cl | |
| 283 | 21 | 36 | 35 | 24 |
| 293 | 32 | 36 | 35 | 37 |
| 313 | 62 | 36 | 40 | 41 |
| 333 | 106 | 37 | 46 | 55 |
| 353 | 167 | 37 | 54 | 66 |
Answer the following questions based on the table given above.
(a) What mass of KNO3 would be needed to produce a saturated solution of KNO3 in 50 grams of water at 313 K.
(b) If a saturated solution of KCl is made at 353 K and then cooled at room temperature, what would you observe? Explain.
(c) Find the solubility of each salt at 293 K.
(d) Which salt has the lowest solubility at 283 K?
(e) What is the effect of change of temperature on the solubility of a salt.
Answer
(a) From the table, the solubility of KNO3 at 313 K is 62 g per 100 g of water.
62 g of KNO3 per 100 g of water
Mass of solute per 50 g of water is
= x 50
= 31 g
∴ Mass of solute per 50 g of water is 31 g.
(b) The solubility of KCl at 353 K is 54 g per 100 g of water.
The solubility of KCl at 293 K (room temperature) is 35 G per 100 g of water.
When the solution cools, the solubility decreases.
The excess KCl will precipitate out of the solution as crystals.
(c) Considering given salts are dissolved in 100g of water
Solubility of KNO3 at 293 K
Mass of solute = 32 g
Mass of solvent = 100 g
Solubility = x 100
= x 100
= 32 g
∴ Solubility of KNO3 at 293 K is 32 g.
Solubility of NaCl at 293 K
Mass of solute = 36 g
Mass of solvent = 100 g
Solubility = x 100
= x 100
= 36 g
∴ Solubility of NaCl at 293 K is 36 g.
Solubility of KCl at 293 K
Mass of solute = 35 g
Mass of solvent = 100 g
Solubility = x 100
= x 100
= 35 g
∴ Solubility of KCl at 293 K is 35 g.
Solubility of NH4Cl at 293 K
Mass of solute = 37 g
Mass of solvent = 100 g
Solubility = x 100
= x 100
= 37 g
∴ Solubility of NH4Cl at 293 K is 37 g.
(d) From the table, when we calculate the solubility of KNO3 at 283 K,
Mass of solute = 21 g
Mass of solvent = 100 g
Solubility = x 100
= x 100
= 21 g
Whereas, the solubility of NaCl, KCl and NH4Cl will be 36 g, 35 g and 24 g respectively.
Hence, the least solubility will be of KNO3 at 283 K
(e) The solubility of most salts in water usually increases with rise in temperature. Example, Potassium nitrate.
There are some salts which show anomalous solubility. Their solubility first increases, and then decreases, with rise in temperature.
Example: Na2SO4.10H2O (Glauber's salt).
Solubility curve of Na2SO4.10H2O rises till it reaches 32.8°C, and then it falls slightly. This is because Na2SO4.10H2O is hydrous below 32.8°C and looses it water and become anhydrous above 32.8°C.
Find the solubility of KNO3 at 20°C when the mass of the empty dish is 50 g. The mass of dish and solution is 65 g, while the mass of dish and residue is 54.3 g.
Answer
Given,
Mass of empty dish (M) = 50 g
Mass of dish and solution (M1 ) = 65 g
Mass of dish and residue (M2 ) = 54.3 g
Mass of saturated solution = M1 - M = 65 - 50 = 15 g
Mass of solute = M2 - M = 54.3 - 50 = 4.3 g
Mass of solvent = mass of saturated solution - mass of solute
= (M1 - M) - (M2 - M)
= 15 - 4.3
= 10.7 g
Solubility = x 100
= x 100
= 40.18 g
∴ Solubility of KNO3 at 20°C is 40.18 g.
What weight of sodium nitrate will separate when a saturated solution containing 50 gram of water is cooled from 50°C to 30°C ? The solubility of NaNO3 at 50°C and 30°C is 114 g and 86 g respectively.
Answer
Given,
Mass of solvent = 50 g
Solubility of NaNO3 at 50°C = 114 g
Solubility of NaNO3 at 30°C = 86 g
Solubility = x 100
Rearranging the above equation
Mass of solute =
∴ Mass of solute at 50°C = x 50
= 57 g
∴ Mass of solute at 30°C = x 50
= 43 g
∴ Amount of NaNO3 that separate = 57 g - 43 g = 14 g
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer
Given,
Mass of solute = 36 g
Mass of solvent = 100 g
Mass of solution = Mass of solute + Mass of solvent
= 36 + 100
= 136 g
Mass percent = x 100
= x 100
= 26.47%
∴ Concentration of sodium chloride in 100 g of water at 293k is 26.47%
Explain the terms
(a) Solution
(b) solute
(c) solvent
Answer
(a) Solution — A solution is a homogenous mixture of two or more components whose composition may be gradually changed by changing the relative amounts of the components.
(b) Solute — The substance that dissolves in the solvent to form a solution is known as Solute.
(c) Solvent — The medium of dissolution that allows one or more components to dissolve in it to form a solution is known as Solvent.
Explain why hot saturated solution of potassium nitrate forms crystals as it cools.
Answer
The solubility of a solution usually decreases with a fall in its temperature. Hence, if the temperature of a saturated solution is lowered, a part of the dissolved solute separates out in the form of crystals.
Give three factors which affect the solubility of a solid solute in a solvent.
Answer
The rate of dissolution or rate of solubility of a solid in a solvent depends on the following factors:
- Size of solute particles — The smaller the size of the solute particles, the greater is its total surface area exposed to the solvent. Therefore, greater is the solubility of that solute.
- Stirring — This brings more of the solvent in contact with the solute and thus increases the rate of formation of solution.
- Temperature — The solubility of a gas in a liquid always decreases with rise in temperature. But the solubility of most solids in water usually increases with rise in temperature.
(a) If you are given some copper sulphate crystals, how would you proceed to prepare its saturated solution at room temperature?
(b) How can you show that your solution is really saturated?
Answer
(a) Preparation of saturated solution of copper sulphate crystals:
Take 100 g of distilled water in a beaker. Add one gram of copper sulphate crystals and stir with a glass rod till the crystals dissolve. Add one more gram of copper sulphate crystals and stir it. It too will dissolve. Continue adding a gram of copper sulphate crystals and stir vigorously after each addition.
A stage is reached when no more copper sulphate dissolves. At this stage, we have a saturated solution of copper sulphate at room temperature.
(b) Add some more copper sulphate crystals to the saturated solution. The crystals do not dissolve no matter how long it is left there or how vigorously it is stirred.
(a) Define
(i) Henry’s law
(ii) Crystallization
(iii) Seeding
(b) State any three methods of crystallization.
Answer
(a) (i) Henry’s law — At any given temperature, the mass of a gas dissolved by a fixed volume of liquid is directly proportional to the pressure on the surface of the liquid.
(ii) Crystallization — It is a process by which crystals of a substance are obtained by cooling a hot saturated solution.
(iii) Seeding — It is a process of inducing crystallization by adding a crystal of a pure substance into its saturated solution.
(b) Three methods of crystallization are:
- By cooling a hot saturated solution gently
- By cooling a fused mass
- By sublimation
What would you observe when crystals of Copper(II) sulphate are heated in a test-tube strongly.
Answer
Drops of colourless liquid condense on the cooler parts of the test tube, leaving behind a residue that is anhydrous (without water) and amorphous (non-crystalline), i.e., with no definite shape or structure.
CuSO4.5H2O ⇌ CuSO4 + 5H2O
Give the names and formulae of two substances in each case :
(a) hydrated substance
(b) anhydrous substance
(c) liquid drying agent
(d) a basic drying agent
Answer
(a) hydrated substance —
- Sodium carbonate decahydrate [Washing soda crystals] : Na2CO3.10H2O
- Copper (II) sulphate pentahydrate [Blue vitriol]: CuSO4.5H2O
(b) anhydrous substance —
- Anhydrous sodium chloride [table salt] : NaCl
- Nitre (KNO3)
(c) liquid drying agent —
- Sulphuric acid: H2SO4
- Phosphoric acid: H3PO4
(d) a basic drying agent —
- Quick lime: CaO
- Potassium carbonate: K2CO3
What is the effect of temperature on solubility of KNO3 and CaSO4 in water?
Answer
KNO3 shows a considerable increase in solubility with rise in temperature.
Solubility of calcium sulphate decreases (after attaining a certain temperature) with further rise in temperature.
Solubility of NaCl at 40°C is 36.5 g. What is meant by this statement
Answer
Solubility of NaCl at 40°C is 36.5 g. means that 36.5 g of NaCl dissolves in 100 g of water at 40°C.
Which test will you carry out to find out if a given solution is saturated or unsaturated or supersaturated?
Answer
Add a few drops of solute in the solution and try to stir by keeping the temperature constant.
- If more solute does not dissolve in the given solution, then it will be a saturated solution.
- If the solute gets dissolved, then it is an unsaturated solution.
- If on slightly disturbing the solution by shaking, stirring, scratching the wall of container or adding a solute crystal to the solution, the additional amount of the solute precipitates out, then the solution is a supersaturated solution.
What is the effect of pressure on solubility of gases? Explain with an example.
Answer
An increase in pressure on the surface of water increases the solubility of a gas in water.
For example: the solubility of carbon dioxide in water under normal atmospheric pressure is rather low, but when the water surface is subjected to higher pressure, a lot more carbon dioxide gas dissolves in it, as is seen in the case of soda water. On opening the soda water bottle, the dissolved gas rapidly bubbles out since pressure on the surface of the water suddenly decreases.
State the term : (Do not give examples)
(a) A solution where solvent is a liquid other than water.
(b) When a substance absorbs moisture on exposure to moist air and dissolves in the absorbed water and turned to solution.
(c) A substance which contains water of crystallisation.
(d) When a substance absorbs moisture from the atmosphere, but does not form a solution.
(e) When a compound loses its water of crystallisation on exposure to dry air.
(f) The substance that can remove hydrogen and oxygen atoms in the ratio of 2 : 1 (in the form of water) from the compounds.
Answer
(a) Non-aqueous solution
(b) Deliquescence
(c) Hydrated substance
(d) Hygroscopy
(e) Efflorescence
(f) Dehydrating agent
Complete the following table :
| Common Name | Chemical Name | Formula | Acid, base or salt | Efflorescent, hygroscopic or deliquescent substance |
|---|---|---|---|---|
| Solid caustic potash | ||||
| Quick lime | ||||
| Oil of vitriol | ||||
| Washing soda | ||||
| Solid caustic soda | ||||
| Blue vitriol |
Answer
| Common Name | Chemical Name | Formula | Acid, base or salt | Efflorescent hygroscopic or deliquescent substance |
|---|---|---|---|---|
| Solid caustic potash | Potassium hydroxide | KOH | Base | Deliquescent |
| Quick lime | Calcium oxide | CaO | Base | Hygroscopic |
| Oil of vitriol | Sulphuric acid | H2SO4 | Acid | Hygroscopic |
| Washing soda | Sodium carbonate decahydrate | Na2CO3.10H2O | Salt | Efflorescent |
| Solid caustic soda | Sodium hydroxide | NaOH | Base | Deliquescent |
| Blue vitriol | Copper (II) Sulphate pentahydrate | CuSO4.5H2O | Salt | Efflorescent |
Explain why :
(a) water is an excellent liquid to use in cooling systems.
(b) a solution is always clear and transparent.
(c) lakes and rivers do not suddenly freeze in the winters.
(d) the solute cannot be separated from a solution by filtration.
(e) fused CaCl2 or conc. H2SO4 is used in a desiccator.
(f) effervescence is seen on opening a bottle of soda water.
(g) Table salt becomes sticky on exposure to humid air during the rainy season.
Answer
(a) Water is an effective coolant. By allowing water to flow in pipes around the heated parts of a machine, heat energy from such parts is removed. Water in pipes can extract more heat from the surroundings without much rise in it's temperature because of it's high specific heat capacity. This is why radiators in car and generator use water for cooling.
(b) Solutions are homogeneous in nature. The solute particles are dispersed uniformly throughout the solvent. The size of particles of a true solution is very small about 10-10 m. Due to homogeneity and small size, they do not scatter light significantly. Hence, a solution is always clear and transparent.
(c) Rivers and lakes do not freeze suddenly because the specific latent heat of fusion of ice is sufficiently high (= 336 J g-1). The water in lakes and rivers will have to liberate a large quantity of heat to the surrounding before freezing. The layer of ice formed over the water surface, being a poor conductor of heat, will also prevent the loss of heat from the water of lake, hence the water does not freeze all at once.
(d) The size of particles of a true solution is about 10-10 m. In a solution, the solute particles and the solvent molecules cannot be distinguished even under a microscope. Due to such small size of particles, the solute cannot be separated from a solution by filtration.
(e) Fused CaCl2 is deliquescent in nature, absorbs moisture and hence used as drying agent or desiccating agent, similarly, conc. sulphuric acid is hygroscopic in nature and can remove moisture from other substances; Hence, they are used as drying agents.
(f) The solubility of carbon dioxide in water under normal atmospheric pressure is rather low, but when the water surface is subjected to higher pressure, a lot more carbon dioxide gas dissolves in it, because, an increase in pressure on the surface of water increases the solubility of the gas in water. On opening the soda water bottle, the dissolved gas rapidly bubbles out since pressure on the surface of the water suddenly decreases. Hence, effervescence is seen on opening a bottle of soda water.
(g) Table salt [sodium chloride] contains impurities like magnesium chloride and calcium chloride, which are deliquescent. Hence, table salt absorb moisture in rainy season and turns sticky.
Normally, solubility of a crystalline solid increases with temperature. Does it increase uniformly in all cases ? Name a substance whose solubility :
(a) increases rapidly with temperature.
(b) increases gradually with temperature.
(c) increases slightly with temperature.
(d) initially increases then decreases with rise in temperature.
Answer
No, solubility of a crystalline solid does not increase with temperature in all cases.
(a) Solubility increases rapidly with temperature — Potassium nitrate
(b) Solubility increases gradually with temperature — Potassium chloride
(c) Solubility increases slightly with temperature — Sodium chloride
(d) Solubility initially increases then decreases with rise in temperature — Calcium sulphate
What are drying or desiccating agents. Give examples.
Answer
Drying or desiccating agents are substances that can readily absorbs moisture from other substances without chemically reacting with them.
Ex: Conc. Sulphuric acid [H2SO4], Phosphorus pentoxide [P2O5], Quicklime [CaO], Silica gel.
In which of the following substances will there be :
(a) increase in mass
(b) decrease in mass
(c) no change in mass when they are exposed to air?
- Sodium chloride
- Iron
- Conc. sulphuric acid
- Table salt
- Sodium carbonate crystals
Answer
(a) Increase in mass: Iron, conc. sulphuric acid, Table salt
Reason — Increase in mass is due to the absorbed water in case of sulphuric acid, Table salt, whereas, gain in mass of iron is due to the increased weight of oxygen which has combined with the iron to form iron oxide or rust.
(b) Decrease in mass: Sodium carbonate crystals
Reason — Decrease in mass is because sodium carbonate loses its water of crystallization on exposure to dry air.
(c) No change in mass: Sodium chloride
Reason — Pure sodium chloride is neither deliquescent nor efflorescent i.e., it does not absorb moisture from atmospheric air nor does it lose it, hence there is no change in mass.
State the methods by which hydrated salts can be made anhydrous?
Answer
Hydrated salts can be made anhydrous by:
- direct heating
- heating in dry or hot air
- heating under vacuum
- by using dehydrating/desiccating agents such as warm concentrated sulphuric acid.
Sodium sulphate is soluble in:
- Ether
- Water
- Alcohol
- Benzene
Answer
Water
Reason — Water has a high dielectric constant, as a result it reduces the electrostatic force of attraction between the positive and negative ions and dissolves even inorganic compounds, which are usually electrovalent. Hence, sodium sulphate is dissolves in water.
Water acts as a universal solvent because:
- It is an organic compound
- It is polar and has a high dielectric constant.
- It is liquid at room temperature.
- It boils at 100°C
Answer
It is polar and has a high dielectric constant.
Reason — Water is a polar covalent compound having a high dielectric constant. This makes water a universal solvent as it helps dissolve even inorganic compounds by reducing the electrostatic force of attraction between the ions.
Permanent hardness of water is removed by:
- Adding calcium sulphate
- Boiling with potassium chloride
- Boiling
- Adding sodium carbonate
Answer
Adding sodium carbonate
Reason — Permanent hardness of water can be removed by addition of washing soda [sodium carbonate]:
Permanent Hard Water:
Solid solutions are called :
- Allotropes
- Isotopes
- Alloys
- Isotones
Answer
Alloys
Reason — A homogenous solution of a solid into another solid is called a solid solution and common metal alloys are solid solutions because an alloy is also a metal made by mixing two types of metals together.
With a rise in temperature, the solubility of sodium chloride will:
- Increase rapidly
- Decrease
- Remain the same
- Increase slightly
Answer
Increase slightly
Reason — In an endothermic process, the solubility of a solute increases with an increase in temperature.
For example: solubility of sodium chloride increases with rise in temperature.
The solubility of which of the following substances decreases with a rise in temperature :
- Potassium chloride
- Hydrated sodium sulphate
- Calcium hydroxide
- Potassium nitrate
Answer
Calcium hydroxide
Reason — In an exothermic process, the solubility increases on lowering the temperature.
For example: solubility of calcium sulphate and calcium hydroxide in water decreases on increasing the temperature.
The crystalline substance that does not contain water of crystallization is:
- Plaster of paris
- Potash alum
- Potassium permanganate
- Epsom salt
Answer
Potassium permanganate
Reason — The crystalline shape of a substance is not necessarily the result of the presence of water of crystallization. In fact, there are number of crystalline solids that crystallize from water without holding any water of crystallization. For example: Potassium permanganate
Table salt becomes sticky in the presence of moisture because :
- It is deliquescent
- It is hygroscopic
- It contains impurities which are deliquescent
- It is a good drying agent.
Answer
It contains impurities which are deliquescent
Reason — Table salt [sodium chloride] contains impurities like magnesium chloride and calcium chloride, which are deliquescent. Hence, table salt absorbs moisture in rainy season and turns sticky.
Which of the following substances can act as a dehydrating as well as a drying agent.
- Oxalic acid
- Conc. nitric acid
- Washing soda
- Conc sulphuric acid
Answer
Conc sulphuric acid
Reason — Being hygroscopic sulphuric acid absorbs moisture but not enough to form a solution. Hence, it is a drying agent.
Sulphuric acid is a dehydrating agent as it can remove chemically combined water molecules from blue vitriol.
CuSO4.5H2O + H2SO4 ⇌ CuSO4 + 5H2O
Which of the following is a deliquescent salt ?
- CuSO4
- FeCl3
- KCl
- ZnSO4
Answer
FeCl3
Reason — FeCl3 absorbs moisture when exposed to the atmosphere and ultimately dissolves in the absorbed water. Hence, it is a deliquescent salt.
If a salt on heating gives water vapour, then that salt is:
- Hygroscopic
- Deliquescent
- Hydrated
- Anhydrous
Answer
Hydrated
Reason — A hydrated salt on heating loses its water of crystallization and becomes anhydrous.
The salt which is the cause of hardness in water:
- Sodium sulphate
- Magnesium bicarbonate
- Sodium chloride
- Calcium nitrate
Answer
Magnesium bicarbonate
Reason — Hardness in water is due to the presence of bicarbonates, chlorides or sulphates of calcium or magnesium.
Temporary hardness of water can be removed by:
- Adding sodium chloride
- Boiling
- Adding calcium carbonate
- Leaving it for a few hours.
Answer
Boiling
Reason — By boiling carbon dioxide is driven off and the soluble hydrogen carbonates are converted into insoluble carbonates and could be removed by filtration or decantation.
Calcium carbonate and magnesium carbonate are precipitated leaving the water soft.
Ca(HCO3)2 CaCO3 ↓ + H2O + CO2 ↑
Mg(HCO3)2 MgCO3 ↓ + H2O + CO2 ↑
The salt which does not contain any water of crystallisation is :
P — Blue vitriol
Q — Gypsum
R — Baking soda
- Only P
- Only Q
- Only R
- Both Q and R
Answer
Only R
Reason — Water of crystallisation is the fixed number of water molecules chemically bound to a salt in its crystalline form. Salts like blue vitriol (CuSO4.5H2O) and gypsum (CaSO4.2H2O) are examples of hydrated salts. But baking soda (NaHCO3) doesn't have any water molecules in its formula. Therefore, it does not contain any water of crystallisation.
Sodium chloride (common salt) besides being used in kitchen can also be used as the raw material for making:
P — Slaked lime
Q — Washing soda
R — Baking soda
- Only P
- Only Q
- Only R
- Both Q and R
Answer
Both Q and R
Reason — Washing Soda (NaHCO3.10H2O) and baking Soda (NaHCO3) are made from sodium chloride using the Solvay process
NaCl + NH3 + CO2 + H2O ⟶ NaHCO3 + NH4Cl
NaHCO3 is filtered and heated to get sodium carbonate:
2NaHCO3 Na2CO3 + CO2 + H2O
Sodium carbonate (Na2CO3) is then hydrated to form washing soda.
The figure shown below demonstrates the solubility curve of a substance. From the statements given below, choose which is/are correct :
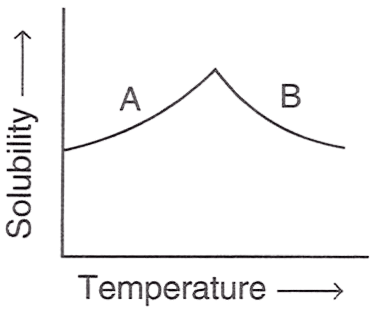
P — A is hydrated sodium chloride, B is anhydrous sodium chloride.
Q — A is Glauber's salt, B is sodium sulphate.
R — A is Gypsum, B is calcium sulphate.
- Only P
- Only Q
- Only R
- Both P and R
Answer
Only R
Reason — A is Glauber's salt and B is sodium sulphate because solubility curve of Na2SO4.10H2O (Glauber's salt) rises till it reaches 32.8°C, and then it falls slightly. This is because Na2SO4.10H2O is hydrous below 32.8°C and anhydrous above it.
Whereas solubility curve of sodium chloride and gypsum are almost flat, showing minimal change in solubility as temperature rises.
Assertion (A): Water is a universal solvent.
Reason (R): Water dissolves all substances except noble metals and glass.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Water dissolves many substances, forming aqueous solutions. Not only solids but gases and other liquids can also dissolve in water to a large extent. For this reason, water is called a universal solvent. Hence the assertion (A) is true.
It is true that nobel metals and glass does not dissolve in water but all compounds do not dissolve in water; for instance, many nonpolar substances, such as oils, do not dissolve in water. Hence reason (R) is false.
Assertion (A): A saturated solution becomes unsaturated on heating.
Reason (R): More amount of solute can dissolve in a solvent upon heating.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — When a saturated solution is heated to a higher temperature, then it becomes unsaturated. More solute can be dissolved in this solution now. Hence, both the assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): A white powder forms on the surface of washing soda crystals which are left exposed to the air.
Reason (R): Washing soda is a hygroscopic substance.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Washing soda (hydrated sodium carbonate), when exposed to dry air, loses water of crystallization. The result is the formation of white anhydrous sodium carbonate (Na2CO3) on the surface, which appears as a white powder. Hence the assertion (A) is true. Washing soda undergoes efflorescence, meaning it loses moisture (its water of crystallization) to the air. So washing soda is efflorescent, not hygroscopic. Hence the reason (R) is false.
Assertion (A): A crusty 'boiler scale' is formed in boilers when hard water is used.
Reason (R): Hard water contains bicarbonates of calcium and magnesium.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Hard water contains bicarbonates of calcium and magnesium. When heated in boilers, these decompose into their respective carbonates, which are insoluble and get deposited as a hard, crusty layer on the inner walls of the boiler, known as boiler scale. Hence, both the assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): Efflorescence is minimum during humid conditions.
Reason (R): Efflorescence occurs when the vapour pressure of the hydrated crystals exceeds the vapour pressure of the atmospheric humidity.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — In humid conditions, the atmospheric vapour pressure increases. Therefore, the difference between the vapour pressure of the hydrated salt and the atmosphere is less, and water does not easily escape from the crystals. Thus, efflorescence is minimized. Hence the assertion (A) is true.
Efflorescence occurs when vapour pressure in the hydrated crystals is higher than atmospheric vapour pressure. Hence reason (R) is true.
Reason (R) explains why efflorescence occur and how vapour pressure of crystal varies according to atmospheric humidity. Hence, reason (R) is correct explanation for assertion (A).
Assertion (A): As the temperature is raised, the amount of solute that can be dissolved in a given quantity of solvent also increases.
Reason (R): A solution which is saturated at a given temperature, becomes unsaturated at a higher temperature.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — When a saturated solution is heated to a higher temperature, the solubility of most solids increases, meaning the solution can now dissolve more solid solute. So, it becomes unsaturated. Hence the assertion (A) is true. As the solution become unsaturated upon heating, more solute can be dissolved in this solution now. Hence the reason (R) is true and it is correct explanation for assertion (A).
Assertion (A): Phosphorus when dissolved in carbon disulphide is a non aqueous solution.
Reason (R): Solutions of substances in a solvent other than water are called non aqueous solutions.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Carbon disulphide is a non-aqueous liquid solvent. Phosphorus when dissolved in carbon disulphide forms a non aqueous solution.
Water is not the only solvent. Alcohol, petrol, ether, benzene, carbon disulphide, liquid ammonia, etc. are some non-aqueous liquid solvents in common use. The solutions made in these liquids are known as non-aqueous solutions. Hence, both the assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): Concentrated sulphuric acid absorbs moisture from the atmosphere.
Reason (R): Conc. H2SO4 is a deliquescent substance.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Concentrated sulphuric acid absorbs moisture (water vapour) from the atmosphere when it is exposed to air but it will not form solution. Such substances are called hygroscopic substances. Hence the assertion (A) is true.
Conc. H2SO4 is hygroscopic substances not deliquescent substance because deliquescent substance absorbs moisture from the air until it dissolves completely in the absorbed water, forming a solution. Hence the reason (R) is false.
Assertion (A): Hardness of water is removed by boiling.
Reason (R): Water contains dissolved calcium and magnesium chlorides.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is false but R is true.
Explanation — Hardness of temporary hard water which contains only hydrogen carbonates of calcium and magnesium can be removed just by boiling. Water containing sulphates and chlorides, of magnesium and calcium is called permanent hard water. This hardness cannot be removed by boiling. Hence the assertion (A) is false.
Water of some springs, wells and rivers contain dissolved mineral matter like hydrogen carbonates, sulphates or chlorides of calcium and magnesium, turning the water hard. Hence the reason (R) is true.
Name:
(a) The solute and solvent in sugar solution.
(b) The characteristic property which makes water the universal solvent.
(c) A substance whose solubility shows an anomalous behaviour.
(d) A substance whose solubility rapidly increases with the temperature.
Answer
(a) Sugar is solute, water is solvent
(b) Water is a polar covalent compound having a dielectric constant which makes it a universal solvent.
(c) Solubility of Glauber's salt [Na2SO4.10H2O] shows anomalous behaviour.
(d) Potassium nitrate
What is the composition of water ? In what volume its elements combine ?
Answer
Water is composed of two atoms of hydrogen and one atom of oxygen in the ratio of 2:1.
Name the substances which give water:
(a) temporary hardness
(b) permanent hardness
Answer
(i) Hydrogen carbonates of calcium and magnesium make water temporary hard.
(ii) Sulphates and chlorides of magnesium and calcium make water permanently hard.
Name the three methods by which hydrous substances can be made anhydrous.
Answer
Hydrous substances can be made anhydrous by:
- Direct heating
- Heating in dry or hot air
- Heating under vacuum
- By using dehydrating/desiccating agents such as warm concentrated sulphuric acid.
What is the use of solubility of oxygen and carbon dioxide in water ?
Answer
Solubility of O2, and CO2 in water is important for the following reasons:
- Marine life like fish use the oxygen of the air dissolved in water for respiration and thus aquatic life is sustained. 1 dm3 (1 litre) of water contains 40 cm3 of dissolved oxygen.
- Aquatic plants make use of dissolved carbon dioxide for photosynthesis, i.e., to prepare their food.
6CO2 + 12H2O C6H12O6 + 6O2 + 6H2O - Carbon dioxide dissolved in water reacts with limestone to form calcium bicarbonate.
CaCO3 + CO2 + H2O ⟶ Ca(HCO3)2
Marine organisms such as snails, oysters, etc., extract calcium carbonate from calcium bicarbonate to build their shells.
Hot saturated solution of sodium nitrate forms crystals, as it cools. Why?
Answer
As solubility of sodium nitrate decreases with decrease in temperature, hence, a part of the dissolved solute separates out in the form of crystals when temperature falls.
What do you understand by:
(a) Soft water
(b) Hard water
(c) Temporary Hard water
(d) Permanent hard water.
Answer
(a) Soft water — Water is said to be soft water if it readily forms lather with soap.
(b) Hard water — Water is said to be hard if it does not readily form lather with soap.
(c) Temporary Hard water — Water that contains only hydrogen carbonates of Calcium and Magnesium is called temporary hard water.
(d) Permanent hard water — Water that contains sulphates and chlorides of Magnesium and Calcium is called Permanent hard water.
What are the causes for
(a) Temporary hardness
(b) Permanent hardness
Answer
(a) The presence of hydrogen carbonates of calcium and magnesium makes water temporarily hard.
(b) The presence of sulphates and chlorides of magnesium and calcium makes water permanently hard.
State two ways, by which a saturated solution can be changed to unsaturated solution.
Answer
A saturated solution can be changed to an unsaturated solution by:
- heating.
- adding more solvent.
What is a soap, what is it used for?
Answer
Soap is chemically a sodium salt of stearic acid (an organic acid with the formula C17H35COOH) and has the formula C17H35COONa (sodium stearate, can be represented by NaSt).
Soap is used for washing & cleaning purposes.
What is the advantage of a detergent over soap ?
Answer
Detergent are more soluble in water than soap and are unaffected by hardness of water as their calcium and magnesium salts are soluble in water so they do not form scum and cleaning action is easily done.
What are hydrous substances? Explain with examples.
Answer
Substances which contain water molecules along with salts like Sodium carbonate decahydrate [Washing soda crystals — Na2CO3.10H2O] and Copper (II) sulphate pentahydrate [Blue vitriol — CuSO4.5H2O] are hydrated substances. This water gives the crystals their shape. In some cases it also gives them their colour (copper sulphate crystals are blue in colour).
What is the importance of dissolved impurities in water ?
Answer
Importance of dissolved impurities in water are as follows:
- Salts and minerals are essential for growth and development of plants.
- They add taste to water.
- They supply the essential minerals needed by our body.
What are the advantages of
(i) soft water
(ii) Hard water
Answer
(i) Advantages of soft water
- With soft water soaps and cleansing agents are consumed less, hence, money is saved.
- Soft water does not leave deposits of minerals on pipes which makes plumbing works easy.
- Clothes washed with soft water lasts long and stay bright.
(ii) Advantages of hard water
- The presence of salts in hard water makes it tasty. It is used in preparation of beverages and wine.
- Calcium and magnesium salts present in small amounts in hard water are essential for the growth of our bones and teeth.
- Hard water checks poisoning of water by lead pipes. When these pipes are used for carrying water, some lead salts dissolve in water to make it poisonous. Calcium sulphate present in hard water forms insoluble lead sulphate in the form of a layer inside the lead pipe and this checks the lead poisoning.
What are stalagmites and stalactites? How are they formed?
Answer
In some limestone caves, sometimes conical pillar-like objects hanging from the roof of the caves and some rising from the floors are seen. These conical pillars which grow upward from the floor of the caves are known as stalagmites, and the structures which grow downwards from the roof are called stalactites.
These are formed by water dripping from the cracks in the rocks containing calcium hydrogen carbonate. Calcium hydrogen carbonate converts to calcium carbonate when pressure is released. Gradually, calcium carbonate deposits both on roof and floor to form stalagmites and stalactites.
Give equations to show what happens when temporary hard water is
(a) boiled
(b) treated with slaked lime
Answer
(a) By boiling carbon dioxide is driven off and the soluble hydrogen carbonates are converted into insoluble carbonates and could be removed by filtration or decantation.
Calcium carbonate and magnesium carbonate are precipitated leaving the water soft.
Ca(HCO3)2 CaCO3 ↓ + H2O + CO2 ↑
Mg(HCO3)2 MgCO3 ↓ + H2O + CO2 ↑
(b) Lime stone is first thoroughly mixed with water in a tank and then fed into another tank containing the hard water. Revolving paddles thoroughly mix the two solutions.
Most of calcium carbonate settles down. If there is any solid left over, it is removed by a filter. This process goes by the name 'Clark's process'.
Ca(HCO3)2 + Ca(OH)2 ⟶ 2CaCO3 ↓ + 2H2O
Mg(HCO3)2 + Ca(OH)2 ⟶ MgCO3 ↓ + CaCO3 ↓ + 2H2O
State the disadvantages of using hard water.
Answer
- Furring of tea kettles is caused by sediment formed from boiling hard water. This fur is carbonates of calcium amd magnesium.
- Hard water is unfit for washing purposes because it is difficult to form lather with soap. Scum may form in a reaction with soap, wasting the soap.
- Hard water is not suitable for producing steam. Solids in hard water incapable of changing into steam get deposited on the inner walls of the tubes. Hence, the tubes become narrower and eventually less steam is produced.
Why does the hardness of water render it unfit for use in a
(i) boiler
(ii) for washing purposes?
Answer
(i) The dissolved substance present in the hard water does not convert into steam and gets deposited on the inner walls of the tube. Hence, the tubes become narrower and eventually less steam is produced.
When bore of the tube becomes very narrow, the pressure of steam increases so much that at times the boiler itself bursts. Hence, hard water is unfit for use in boilers.
(ii) If the water is hard, calcium and magnesium ions of the water combine with the negative ions of the soap to form a slimy precipitate of insoluble calcium and magnesium usually called soap curd (scum).
Formation of soap curd will go on as long as calcium and magnesium ions are present. Till then, no soap lather will be formed and cleaning of clothes or body will not be possible. Moreover, these precipitates are difficult to wash from fabrics and sometimes form rusty spots if iron salts are present in water.
Explain with equation, what is noticed when permanent hard water is treated with
(a) slaked lime
(b) washing soda
Answer
(a) In order to treat permanent hard water, slaked lime is used, magnesium hydroxide and calcium carbonate precipitates out and can be easily filtered.
MgSO4 + Na2CO3 + Ca(OH)2 ⟶ Mg(OH)2 ↓ + CaCO3 ↓ + Na2SO4
(b) Washing soda is added to hard water, which results in settling down of insoluble carbonates which can be removed by filtration.
What is permutit method, how can it be used for softening hard water ?
Answer
Permutit is an artificial zeolite. Chemically, it is hydrated sodium aluminium orthosilicate, having the formula Na2Al2Si2O8.XH2O. For the sake of convenience, let us give it the formula Na2P.
A tall cylinder is loosely filled with lumps of permutit. When hard water containing calcium and magnesium ions percolates through these lumps, exchange of ions takes place. The sodium permutit is slowly changed into calcium and magnesium permutit, and with the removal of calcium and magnesium ions, the water become soft.
When no longer active, the permutit is regenerated by running a concentrated solution of brine over it and removing the calcium chloride formed by repeated washing.
CaP + 2NaCl ⟶ Na2P + CaI2
Explain, with equations, why ordinary soap does not lather easily in hard water.
Answer
If the water is hard, calcium and magnesium ions of the water combine with the negative ions of the soap to form a slimy precipitate of insoluble calcium and magnesium usually called soap curd (scum).
In permanent hard water, the formation of soap- curd will go on as long as there are calcium and magnesium ions present.
As long as the formation of soap-curd continues, no soap lather will be formed and the cleaning of cloth or body will not be possible.
Explain:
(a) The use of lead pipes for drinking water supply is being discontinued.
(b) Chalk hills dissolve in rain water.
(c) Hard water is unfit for boilers.
(d) Iron chloride forms a saturated solution when exposed to the atmosphere.
(e) A bottle containing concentrated H2SO4 should be stoppered.
Answer
(a) Lead is toxic. When water flows through lead pipes, especially soft or acidic water, it can dissolve lead ions into the water. Ingesting lead causes serious health issues, especially in children (like brain and kidney damage).
(b) Chalk is mainly calcium carbonate (CaCO3). Rainwater absorbs carbon dioxide (CO2) from the air and forms carbonic acid (H2CO3), a weak acid:
CO2 + H2O ⟶ H2CO3
This reacts with calcium carbonate:
H2CO3 + CaCO3 ⟶ Ca(HCO3)2
The product, calcium bicarbonate, is soluble in water, so the chalk slowly dissolves.
(c) The dissolved substance present in the hard water does not convert into steam and gets deposited on the inner walls of the tube. Hence, the tubes become narrower and eventually less steam is produced.
When bore of the tube becomes very narrow, the pressure of steam increases so much that at times the boiler itself bursts. Hence, hard water is unfit for use in boilers.
(d) Ferric chloride (FeCl3) is deliquescent, it absorbs moisture from the air and dissolves in the water it absorbs, eventually forming a saturated solution. This property makes it unstable in open air.
(e) Concentrated sulphuric acid H2SO4 is highly hygroscopic, meaning it absorbs water vapour from the air. If left open, it will absorb moisture and become diluted. To maintain concentration and prevent hazards, it should always be tightly stoppered.
The following figure shows the solubility curves of NaCl, KNO3 and hydrated calcium sulphate.

(a) Identify and label the curves with the salt it represents.
(b) State the factors on which the solubility depends.
(c) Solubility of which salt(s) shows :
(i) Endothermic process
(ii) Exothermic process?
Answer
(a) The labelled curves are shown below:

(b) Solubility of a solid in a solvent depends on the following factors:
- Size of solute particles — The smaller the size of the solute particles, the greater is its total surface area exposed to the solvent. Therefore, greater is the solubility of that solute.
- Stirring — This brings more of the solvent in contact with the solute and thus increases the rate of formation of solution.
- Temperature — The solubility of a gas in a liquid always decreases with rise in temperature. But the solubility of most solids in water usually increases with rise in temperature.
In case of NaCl, solubility increases only a little with increase in temperature.
In case of KNO3, solubility increases considerably with increase in temperature.
In case of calcium sulphate, solubility decreases (after attaining a certain temperature) with further rise in temperature.
(c) (i) In an endothermic process, the solubility of a solute increases with an increase in temperature.
For example: solubility of KNO3 increases with rise in temperature and solubility of NaCl increases only a little with increase in temperature.
(ii) In an exothermic process, the solubility increases on lowering the temperature.
For example: solubility of calcium sulphate in water decreases on increasing the temperature.