(a) What is a chemical reaction?
(b) State the conditions necessary for a chemical change or reaction.
Answer
(a) A chemical reaction is a process of breaking chemical bonds of the reacting substances (reactants) and making new bonds to form new substances (products).
(b) Conditions necessary for a chemical change or reaction are :
Mixing (close contact) — Some chemical reactions take place when two substances are mixed in their solid state.
Pb(NO3)2 (s) + 2KI (s) ⟶ 2KNO3 + PbI2 (s)Solution — In some cases, a chemical reaction occurs when substances are mixed either in molten or aqueous state.
AgNO3 + NaCl ⟶ AgCl ↓ + NaNO3Heat — Some chemical reactions occur only on heating.
CuCO3 (s) CuO (s) + CO2 ↑ (g)Light — Some chemical reactions take place by the action of light. They are called photochemical reaction.
2AgNO3 2Ag + 2NO2 + O2 ↑Electricity — Some reactions are caused or accompanied by the passage of an electric current. They are called electrochemical reaction.
2NaCl 2Na + Cl2 ↑Pressure — Some chemical reaction take place only when the involved substances are subjected to high pressure.
N2 + 3H2 2NH3 ↑Catalyst — Some chemical reactions need a catalyst to accelerate or decelerate the rate at which they occur.
2H2O2 2H2O + O2 ↑
Define the following terms:
(a) Chemical change
(b) Chemical bond
(c) Effervescence
(d) Precipitate
Answer
(a) A chemical change is a permanent change in which the chemical composition of a substance is changed and one or more new substances with different chemical compositions and different properties are formed.
(b) A chemical bond is the force that holds the atoms of a molecule together, as in a compound.
(c) Effervescence is the formation of gas bubbles in a liquid during a reaction.
(d) Certain chemical reactions are characterized by the formation of insoluble solid substances called precipitate.
Give an example of a reaction where the following are involved:
(a) Heat
(b) Light
(c) Electricity
(d) Close contact
(e) Solution
(f) Pressure
(g) Catalyst
Answer
(a) Heat
CuCO3 (s) CuO (s) + CO2 ↑ (g)
(b) Light
2AgNO3 2Ag + 2NO2 ↑ + O2 ↑
(c) Electricity
2NaCl 2Na + Cl2 ↑
(d) Close contact
Pb(NO3)2 (s) + 2KI (s) ⟶ 2KNO3 + PbI2 (s)
(e) Solution
NaCl (aq.) + AgNO3 (aq.) ⟶ AgCl (white ppt.) ↓ + NaNO3 (aq.)
(f) Pressure
N2 + 3H2 2NH3 ↑
(g) Catalyst
2H2O2 2H2O + O2 ↑
Define :
(a) Photochemical reaction
(b) Electrochemical reaction.
Give one example in each case.
Answer
(a) A chemical reaction that takes place by the action of light is called a photochemical reaction.
Example —
2AgNO3 2Ag + 2NO2 ↑ + O2 ↑
(b) Reactions that are caused or accompanied by the passage of an electric current are called electrochemical reaction.
Example — On passing current through through molten sodium chloride, sodium and chloride are obtained separately.
2NaCl 2Na + Cl2 ↑
Give an example of each of the following chemical changes.
(a) A photochemical reaction involving 1. silver salt 2. water
(b) A reaction involving 1. blue solution 2. formation of a dirty green precipitate
(c) Two gases combine to form a white solid.
(d) Two solids combine to form a liquid.
(e) A reaction where color change is noticed.
Answer
(a) A photochemical reaction involving 1. silver salt
2AgNO3 2Ag + 2NO2 ↑ + O2 ↑ 2. water
Cl2 + H2O HCl + HClO
(b) A reaction involving 1. blue solution
2. formation of a dirty green precipitate
(c) Two gases combine to form a white solid.
NH3 (g) + HCl (g) ⟶ NH4Cl (s)
(d) Two solids combine to form a liquid.
C (s) + 2S (s) ⟶ CS2 (l)
(e) A reaction where color change is noticed.
Write the chemical reaction where the following changes are observed.
(a) Gas is evolved
(b) Colour change is noticed
(c) Precipitate is formed
(d) Physical state of reactants is changed
Answer
(a) Gas is evolved
Zn(s) + H2SO4 (aq) ⟶ ZnSO4 (aq) + H2 (g)
(b) Colour change is noticed
(c) Precipitate is formed
(d) Physical state of reactants is changed
C (s) + 2S (s) ⟶ CS2 (l)
Give reason for the following :
(a) Silver nitrate solution is kept in coloured bottles.
(b) Molybdenum is used in the manufacture of ammonia.
(c) Blue solution of copper sulphate changes to green when a piece of iron is added to this solution.
(d) Colourless concentrated sulphuric acid in a test tube changes to blue on adding a small piece of copper to it.
Answer
(a) Silver nitrate solution is kept in brown bottles in the laboratory because it decomposes in the presence of light. It becomes black and forms silver, nitrogen dioxide and oxygen.
2AgNO3 2Ag + 2NO2 ↑ + O2 ↑
(b) Molybdenum acts as a promoter and increases the efficiency of the catalyst iron, hence it is used in the manufacture of ammonia.
(c) When a piece of iron is added to a blue coloured copper sulphate solution, the blue colour of the solution fades and eventually turns into light green due to the formation of ferrous sulphate.
(d) When a small piece of copper is added to concentrated sulphuric acid, a blue solution of copper sulphate is formed.
State the type of reactions and give one equation in each case.
(a) Two or more reactants combine to form a single product.
(b) A single reactant breaks up into two or more products.
Answer
(a) Combination reaction or Synthesis
2SO2 (g) + O2 (g) ⟶ 2SO3(s)
(b) Decomposition reaction
2H2O (l) 2H2 (g) + O2 (g)
Give an example of each:
Combination reaction in which:
(i) An element of valency 3 combines with nitrogen.
(ii) A non-metal oxide reacting with water to form an acid.
(iii) A metal oxide reacting with water to form its hydroxide.
Answer
(i) 2Al + N2 ⟶ 2 AlN
(ii) SO2 + H2O ⟶ H2SO3 (sulphurous acid)
(iii) K2O + H2O ⟶ 2KOH (Caustic potash)
Give an example of each:
Write the decomposition reactions for the following:
(i) Metallic oxide on heating forms metallic oxide and oxygen.
(ii) A compound on heating gives a gas which turns lime water milky.
(iii) A white hydroxide on heating gives a yellow residue and water vapours.
Answer
(i)
(ii) ZnCO3 ⟶ ZnO + CO2 ↑
(iii) Zn(OH)2 ⟶ ZnO + H2O
Write the reaction for the decomposition of a nitrate which does not yield nitrogen dioxide on heating.
Answer
2KNO3 ⟶ 2KNO2 + O2
Give two balanced equations to show thermal dissociation.
Answer
Two balanced equations showing thermal dissociation:
NH4Cl ⇌ NH3 ↑ + HCl ↑
N2O4 2NO2
Name:
(a) two coloured oxides that do not decompose on heating.
(b) a carbonate that is stable to heat
(c) an oxide formed during thunder and lighting.
(d) the compound formed when phosphorus and oxygen combines.
Answer
(a) Two coloured oxides that do not decompose on heating are
- Copper(II) oxide (CuO) – Black
- Iron(III) oxide (Fe2O3) – Reddish-brown
(b) Potassium Carbonate (K2CO3)
(c) Nitric oxide (NO)
(d) Phosphorus pentoxide (P2O2)
Which of the following is not a characteristic of a chemical change?
It is irreversible
No net energy change is involved
New substance is formed
Involves absorption or liberation of energy
Answer
No net energy change is involved
Reason — Characteristics of a chemical change are :
It's irreversible.
A new substance is formed.
Involves absorption or liberation of energy.
A reaction of a type: AB + CD ⟶ AD + CB, involves
no chemical change
decomposition of AB and CD
exchange of ions of AB and CD
combination of AB and CD
Answer
exchange of ions of AB and CD
Reason — In the type of reaction called double displacement two compounds exchange their positive and negative radicals.
Example : AB + CD ⟶ AD + CB
The reaction:
BaCl2 (aq) + H2SO4 (aq) ⟶ BaSO4 (s) + 2HCl (aq) is
displacement reaction
neutralisation reaction
decomposition reaction
double displacement reaction
Answer
Double displacement reaction
Reason — A chemical reaction in which both reactants [compounds] are decomposed to give two new compounds by exchanging their radicals is called a Double decomposition or double displacement reaction.
It is represented as XY + AB ⟶ XB + AY
Thermal decomposition of sodium carbonate will produce
carbon dioxide
oxygen
sodium hydroxide
no other product
Answer
Carbon dioxide
Reason — Sodium carbonate, on heating gives sodium oxide and carbon dioxide
Na2CO3 Na2O + CO2 ↑
Complete the following statements.
(a) The chemical change involving iron and hydrochloric acid illustrates a ............... reaction.
(b) In the type of reaction called ............... two compounds exchange their positive and negative radicals.
(c) A catalyst either ............... or ............... the rate of a chemical change but itself remains ............... at the end of the reaction.
(d) On heating, hydrated copper sulphate changes its colour from ............... to ...............
Answer
(a) The chemical change involving iron and hydrochloric acid illustrates a displacement reaction.
(b) In the type of reaction called double displacement two compounds exchange their positive and negative radicals.
(c) A catalyst either accelerates or decelerates the rate of a chemical change but itself remains unaffected at the end of the reaction.
(d) On heating, hydrated copper sulphate changes its colour from blue to white.
Match the following :
| (a) Zn(s) + H2SO4(aq) ⟶ ZnSO4(aq) + H2(g) | (i) Photochemical decomposition |
| (b) 2AgCl(s) 2Ag(s) + Cl2(g) | (ii) Thermal decomposition |
| (c) 2KCl 2K + Cl2 | (iii) Displacement reaction |
| (d) 2HgO(s) 2Hg(s) + O2 | (iv) Electrolytic decomposition |
Answer
| (a) Zn(s) + H2SO4(aq) ⟶ ZnSO4(aq) + H2(g) | (iii) Displacement reaction |
| (b) 2AgCl(s) 2Ag(s) + Cl2(g) | (i) Photochemical decomposition |
| (c) 2KCl 2K + Cl2 | (iv) Electrolytic decomposition |
| (d) 2HgO(s) 2Hg(s) + O2 | (ii) Thermal decomposition |
When hydrogen burns in oxygen, water is formed; when electricity is passed through water, hydrogen and oxygen are given out. Name the type of chemical changes involved in the two cases.
Answer
When hydrogen burns in oxygen, hydrogen and oxygen combine together and forms water, hence, it is a direct combination reaction whereas, when electricity is passed through water, it breaks to give hydrogen and oxygen, hence, it is a decomposition reaction.
Explain the following chemical changes by giving one example of each:
(a) Double decomposition
(b) Thermal dissociation
(c) Reversible reaction
(d) Displacement
Answer
(a) Double decomposition — This is a type of chemical change in which two compounds in a solution react to form two new compounds by mutual exchange of radicals. Double decomposition reaction is also called double displacement reaction.
AB + CD ⟶ AD + CB
Eg., AgNO3 + NaCl ⟶ AgCl ↓ + NaNO3
(b) Thermal dissociation — A reversible decomposition reaction brought about only by heat is called thermal dissociation reaction.
Heat some solid ammonium chloride in a test tube. Two colourless gases, ammonia and hydrogen chloride, are produced. As these gases move up to the upper part of the test tube which is cooler, they combine to form ammonium chloride, which appears as a white sublimate on the upper cooler side of the test tube.
NH4Cl ⇌ NH3 ↑ + HCl ↑
(c) Reversible reaction — A reaction that can be reversed by changing the conditions under which the reaction is taking place is called a reversible reaction.
(d) Displacement — A chemical change in which a more active element displaces a less active element from its salt solution is called a displacement reaction.
Mg + H2SO4 ⟶ MgSO4 + H2 ↑
(a) What is synthesis?
(b) What kind of chemical reaction is synthesis? Support your answer with an example.
Answer
(a) A reaction in which two or more substances combine together to form a single substance is called a synthesis or combination reaction.
A + B ⟶ AB
(b) Synthesis is a direct combination reaction.
In the above reaction, substances A and B combine to give a molecule of a new substance, AB (Product).
For example, carbon burns in oxygen to form a gaseous compound, carbon dioxide.
C + O2 CO2 ↑
Decomposition brought about by heat is known as thermal decomposition. What is the difference between thermal dissociation and thermal decomposition?
Answer
Difference between the two is that thermal dissociation is a reversible reaction whereas, thermal decomposition is an irreversible reaction.
(a) Define neutralization reaction with an example.
(b) Give a balanced equation for this reaction.
(c) Give three applications of neutralization reactions.
Answer
(a) The reaction between an acid and a base that forms salt and water only is referred to as Neutralization reaction.
Reaction between an acid and an alkali to form salt and water only is an example of neutralization reaction.
(b) NH4OH + HCl ⟶ NH4Cl + H2O
(c) Applications of neutralization reactions are as follows :
- When someone is stung by a bee, formic acid enters the skin and causes pain, which can be relieved by rubbing the spot with slaked lime or baking soda, both of which are bases and thus neutralize the acid.
- The acid which is accidentally spilt on to our clothes can be neutralised with ammonia solution.
- If soil is somewhat acidic and thus unfavourable for the growth of certain crops, slaked lime is added to neutralise the excess acid.
What do you understand by precipitation reaction? Explain with an example.
Answer
A chemical reaction in which two compounds in their aqueous state react to form an insoluble salt (precipitate) as one of the products is called a precipitation reaction.
Example: Take a solution of silver nitrate in a test tube and add dil. hydrochloric acid. A curdy white ppt. is formed.
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
(a) What are double displacement reactions?
(b) Give an example of a double displacement reaction, where gas is evolved.
Answer
(a) Double displacement reactions are those in which two compounds in a solution react to form two new compounds by mutual exchange of radicals. This type of reaction is also known as a double decomposition reaction.
(b) FeS (s) + H2SO4 (aq) ⟶ FeSO4 (aq) + H2S ↑
(a) What is a decomposition reaction ?
(b) Decomposition reactions can occur by (i) heat (ii) electricity and (iii) sunlight.
Give two balanced reactions for each.
Answer
(a) The chemical reaction in which a compound splits into two or simpler substances (elements or compounds) is called decomposition reaction.
(b) Two balanced chemical equations for:
(i) Heat
2HgO (s) 2Hg (l) + O2 (g)
CaCO3 (s) CaO (s) + CO2 (g)
(ii) Electricity
2NaCl (l) 2Na (l) + Cl2 (g)
2H2O (l) 2H2 (g) + O2 (g)
(iii) Light
2AgCl (s) 2Ag (s) + Cl2 (g)
2H2O2 (l) 2H2O (l) + O2 (g)
State the type of reactions each of the following represent and balance the ones that are not balanced.
(a) Cl2 + 2KBr ⟶ 2KCl + Br2
(b) NaOH + HCl ⟶ NaCl + H2O
(c) 2HgO ⟶ 2Hg + O2 ↑
(d) Fe + CuSO4 ⟶ FeSO4 + Cu
(e) PbO2 + SO2 ⟶ PbSO4
(f) 2KClO3 ⟶ 2KCl + 3O2 ↑
(g) 2H2O2 ⟶ 2H2O + O2 ↑
(h) KNO3 + H2SO4 ⟶ HNO3 + KHSO4
(i) CuO + H2 ⟶ Cu + H2O
(j) CaCO3 ⟶ CaO + CO2 ↑
(k) NH4Cl ⟶ NH3 + HCl
(l) PbO + 2HNO3 ⟶ Pb(NO3)2 + 2H2O
(m) AgNO3 + NaCl ⟶ AgCl ↓ + NaNO3
Answer
(a) Cl2 + 2KBr ⟶ 2KCl + Br2
Displacement reaction
(b) NaOH + HCl ⟶ NaCl + H2O
Neutralisation reaction
(c) 2HgO ⟶ 2Hg + O2 ↑
Decomposition reaction
(d) Fe + CuSO4 ⟶ FeSO4 + Cu
Displacement reaction
(e) PbO2 + SO2 ⟶ PbSO4
Combination reaction
(f) 2KClO3 ⟶ 2KCl + 3O2 ↑
Decomposition reaction
(g) 2H2O2 ⟶ 2H2O + O2 ↑
Decomposition reaction
(h) KNO3 + H2SO4 ⟶ HNO3 + KHSO4
Double decomposition reaction
(i) CuO + H2 ⟶ Cu + H2O
Displacement reaction
(j) CaCO3 ⟶ CaO + CO2 ↑
Decomposition reaction
(k) NH4Cl ⟶ NH3 ↑ + HCl
Decomposition reaction
(l) PbO + 2HNO3 ⟶ Pb(NO3)2 + H2O
Neutralisation reaction
(m) AgNO3 + NaCl ⟶ AgCl ↓ + NaNO3
Double decomposition reaction
Which of the of following is not a chemical change.
- Rusting of iron
- Iron is magnetised
- Potassium is dissolved in water
- Magnesium wire is burnt
Answer
Iron is magnetised
Reason — Magnetisation of iron is not a chemical change, as it is a temporary change.
Heating of zinc nitrate is a ............... reaction.
- Displacement
- Combination
- Decomposition
- Redox
Answer
Decomposition reaction
Reason — Zinc nitrate undergoes thermal decomposition and produces reddish brown Nitrogen dioxide gas (NO2), Zinc oxide (ZnO), and Oxygen gas (O2).
Burning of a metal is an example of :
- Decomposition reaction
- Combination reaction between an element and a compound
- Direct combination reaction between two elements
- Displacement reaction
Answer
Direct combination reaction between two elements
Reason — A reaction in which two elements react to form a single substance is called a direct combination reaction between two elements.
When a metal, for example Magnesium, is burnt, it reacts with oxygen to form a new compound magnesium oxide.
2Mg + O2 ⟶ 2MgO
Hence, burning of a metal is a direct combination reaction between two elements.
Formation of white precipitate on mixing sodium chloride solution and silver nitrate solution is an example of ............... reaction.
- Combination
- Decomposition
- Displacement
- Double decomposition
Answer
Double decomposition
Reason — A chemical reaction in which both reactants [compounds] are decomposed to give two new compounds by exchanging their radicals is called a Double decomposition or double displacement reaction. Eg.,
AgNO3 + NaCl ⟶ AgCl ↓ + NaNO3
A brown substance A on heating in air turns black forming another substance B. Substances A and B are:
- A = Fe and B = FeO
- A = Zn and B = ZnO
- A = Cu and B = CuO
- A = Pb and B = PbO
Answer
A = Cu and B = CuO
Reason — Brown copper on heating forms black copper oxide.
2Cu + O2 ⟶ 2CuO
Which one of these is a chemical change?
- Water changes to steam
- Dissolution of sugar in water
- Combustion of LPG
- Liquefying ammonia
Answer
Combustion of LPG
Reason — A chemical change is a permanent change and combustion of LPG is a permanent change.
The reaction of neutralization is a :
- Displacement reaction
- Double decomposition
- Combination reaction
- Combination reaction between two compounds
Answer
Double decomposition
Reason — A chemical reaction in which both reactants [compounds] are decomposed to give two new compounds by exchanging their radicals is called a Double decomposition or double displacement reaction.
These reactions are of two types:
- precipitation reaction
- neutralization reaction
FeS + H2SO4 ⟶ FeSO4 + H2S is an example of:
- Displacement reaction
- Double decomposition reaction
- Decomposition reaction
- Combination reaction
Answer
Double decomposition reaction
Reason — A chemical reaction in which both reactants [compounds] are decomposed to give two new compounds by exchanging their radicals is called a Double decomposition or double displacement reaction.
AB + CD ⟶ AD + CB
Which of the following statements is/are correct with respect to the figure shown alongside?
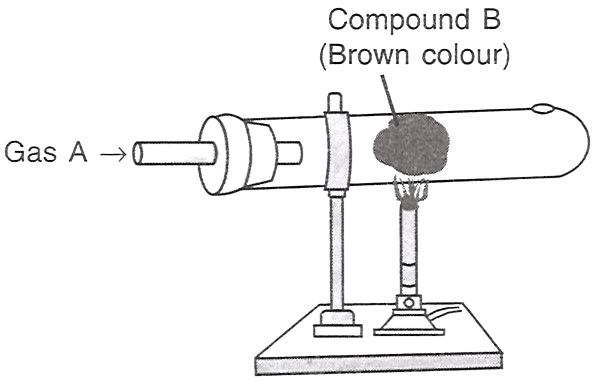
P — A is HCl, B is iron.
Q — A is Cl2, B is copper.
R — A is Cl2, B is iron.
Only P
Only Q
Only R
Both P and R
Answer
Only R
Reason — When chlorine gas (Gas A) is passed over heated iron (Compound B), it forms Iron(III) chloride, which is brown in color.
Whereas hydrochloric acid (HCl) will not produce the same reaction, and copper reacting with chlorine gas will produce copper(II) chloride, which is typically green or blue-green, not brown.
Hence A is chlorine gas and B is iron.
Aman performed the following four experiments
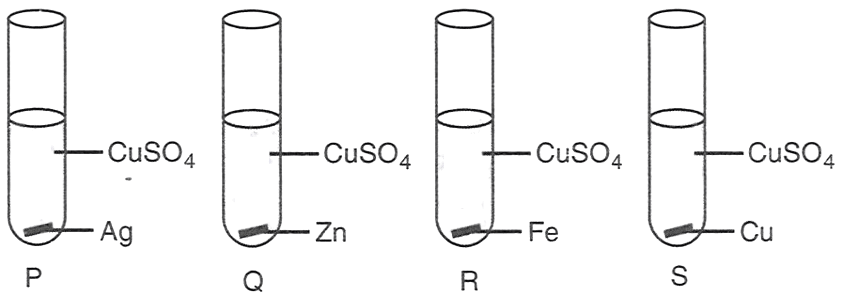
The experiments in which the blue colour of the solution will fade are :
P and Q
P, Q and R
Q and R
Q, R and S
Answer
Q and R
Reason — Blue colour is because of CuSO4. In experiment Q, zinc displaces copper from copper sulphate solution because zinc is more reactive and present above copper in reactivity series.
CuSO4 (aq) + Zn ⟶ ZnSO4 + Cu ↓
In experiment R, iron displaces copper from copper sulphate solution because iron is also more reactive and present above copper in reactivity series.
Hence, in experiment Q and R, copper is displaced by more reactive metal. So, the blue colour of the solution will fade.
The figure given below demonstrates the preparation of oxygen.
2KClO3 (s) 2KCl (s) + 3O2 (g)
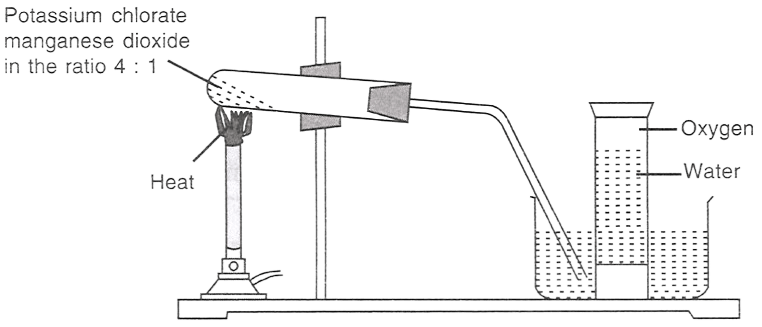
(I) Which of the following statements is/are correct about the reaction?
It is a decomposition reaction and is exothermic.
It is a decomposition reaction and is endothermic.
It is a photochemical decomposition reaction.
It is a combination reaction.
(II) Potassium chlorate is:
an oxidising agent.
a reducing agent.
both reducing as well as an oxidising agent.
all options are correct.
(III) In the reaction, manganese dioxide:
takes part in the reaction.
acts as a catalyst.
decreases the speed of reaction.
helps in producing more oxygen.
Answer
(I) It is a decomposition reaction and is endothermic.
Reason — When potassium chlorate (2KClO3) is heated in the presence of manganese dioxide it will undergo decomposition reaction. Since heat is absorbed during the process it is a endothermic reaction.
(II) an oxidising agent
Reason — Potassium chlorate (KClO3) provides oxygen, which supports oxidation of other substances. It releases oxygen, hence it can oxidize other materials.
(III) acts as a catalyst.
Reason — Potassium chlorate decomposes only at 700°C and even then the rate of release of oxygen is very slow. But when potassium chlorate is heated in the presence of manganese dioxide, decomposition begins at a much lower temperature, 300°C and manganese dioxide remains unaffected. Thus, in this reaction, manganese dioxide acts as a catalyst
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
P — The temperature of the solution increases.
Q — The temperature of the solution decreases.
R — The temperature of the solution remains the same.
S — Salt formation takes place.
Only P
P and R
Q and R
P and S
Answer
P and S
Reason — When a solution of an acid is mixed with a solution of a base, it forms salt and water. This is referred to as neutralization reaction. Neutralization reactions are also exothermic reactions. Hence the temperature of the solution increases.
For example :
NaOH + HCl ⟶ NaCl + H2O + Heat
Assertion (A): Zinc can displace copper from aqueous copper sulphate solution.
Reason (R): Copper is placed above zinc in the reactivity series.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Zinc is more reactive than copper. So, zinc is placed above copper in reactivity series. Hence, zinc displaces copper from aqueous copper sulphate solution. Hence the assertion (A) is true.
CuSO4 (aq) + Zn → ZnSO4 + Cu ↓
Copper is less reactive than zinc and is placed below zinc in reactivity series. Less reactive elements cannot displace more active elements. Hence, copper cannot displace zinc from its salt solution. Hence the reason (R) is false.
Assertion (A): In a redox reaction, the electron gaining species acts as a reducing agent.
Reason (R): Oxidation and reduction reactions occur simultaneously in redox reaction.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is false but R is true.
Explanation — The electron gaining species is being reduced, so it acts as an oxidizing agent, not as a reducing agent. The reducing agent is the one that loses electrons. Hence the assertion (A) is false.
A redox reaction is a chemical reaction where both oxidation and reduction occur simultaneously. Hence the reason (R) is true.
Assertion (A): The decomposition of vegetable matter into compost is an endothermic reaction.
Reason (R): Decomposition reactions involve the breakdown of a single reactant into simpler products.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is false but R is true.
Explanation — The decomposition of vegetable matter (also called composting) is an exothermic reaction. Microbial activity breaks down the organic material and releases heat. Hence the assertion (A) is false.
A chemical reaction in which a compound splits into two or more simpler substances (elements or compounds) is called decomposition reaction. Hence the reason (R) is true.
Assertion (A): The decomposition of silver bromide is utilized in black and white photography.
Reason (R): Light provides energy for this endothermic reaction.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Silver bromide (AgBr) decomposes when exposed to light. The formation of black metallic silver is what creates the image in black and white photography. Hence the assertion (A) is true.
2AgBr 2Ag + Br
Above reaction is an endothermic reaction where heat is absorbed. Light provides energy for this decomposition of silver bromide. Hence the reason (R) is true and reason (R) is a correct explanation of assertion (A).
Assertion (A): Chlorine can displace iodide ions from KI solution.
Reason (R): It is an example of displacement reaction.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true but R is not the correct explanation of A.
Explanation — Chlorine is more active non-metal when compared to iodine. In the reactivity series of non-metals, chlorine is placed above iodine. Hence, chlorine displaces iodine from potassium iodide solution. Hence, the assertion (A) is true.
2KI + Cl2 ⟶ 2KCI + I2
Above reaction is displacement reaction in which a more active element chlorine displaces a less active element iodine from its salt solution. Hence the reason (R) is true.
However reason (R) does not explain about reactivity of chlorine and position of chlorine and iodine in reactivity series. Hence reason (R) is not a correct explanation of assertion (A).
Assertion (A): Silver is displaced by copper from the aqueous solution of silver nitrate.
Reason (R): Copper is placed below silver in the activity series.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Silver is displaced by copper from the aqueous solution of silver nitrate because copper is more reactive than silver. Hence the assertion is true.
Copper is placed above silver in the activity series. So copper is more reactive and can displace silver. Hence the reason (R) is false.
Name
(a) a carbonate which does not decompose on heating.
(b) a nitrate which produces oxygen as the only gas.
(c) a compound which produces carbon dioxide on heating
(d) a nitrate which produces brown gas on heating.
Answer
(a) Sodium carbonate
(b) Potassium nitrate
(c) Calcium carbonate
(d) Lead nitrate
Which is the most reactive element in this question :
CuSO4 + Fe ⟶ FeSO4 + Cu
Answer
Fe, as Fe is higher in the metal reactivity series. The reactivity of the metal reactivity series decreases as one moves from top to bottom.
Define :
(a) Photochemical reaction
(b) Electrochemical reaction
Give one example in each case.
Answer
(a) Photochemical reaction — It is a reaction that occurs with the absorption of light.
Example : Decomposition of silver nitrate
2AgNO3 2Ag + 2NO2 ↑ + O2 ↑
(b) Electrochemical reaction — It is a reaction that occurs with absorption of electrical energy.
Example : Acidulated water breaks into hydrogen and oxygen when electric current is passed through it.
2H2O 2H2 ↑ + O2 ↑
Give an example of a reaction where the following are involved
(a) Evolution of heat
(b) Absorption of heat
(c) High pressure is required
Answer
(a) CH4 + 2O2 ⟶ CO2 ↑ + 2H2O + Heat
(b) C + 2S CS2
(c) N2 + 3H2 2NH3 ↑
Why does the blue colour of copper sulphate solution fade on adding iron fillings ?
Answer
Blue colour of copper sulphate fades away as iron displaces copper from copper sulphate solution as iron is more reactive than copper.
Give an example of:
(a) Single displacement reaction
(b) Double displacement reaction
(c) Photochemical reaction
(d) Thermal decomposition reaction
(e) Electrolytic decomposition reaction
(f) Combination reaction between two compounds ?
Answer
(a) Single displacement reaction
CuSO4 + Zn ⟶ Cu + ZnSO4
(b) Double displacement reaction
BaCl2 (aq) + H2SO4 (aq) ⟶ BaSO4 (s) + 2HCl (aq)
(c) Photochemical reaction
2AgNO3 2Ag + 2NO2 ↑ + O2 ↑
(d) Thermal decomposition reaction
2HgO (s) 2Hg (l) + O2 (g)
(e) Electrolytic decomposition reaction
2H2O 2H2 + O2 ↑
(f) Combination reaction between two compounds ?
NH3 + HCl ⟶ NH4Cl
What do you understand by a 'chemical reaction'?
Answer
A chemical reaction is a process of breaking chemical bonds of the reacting substances (reactants) and making new bonds to form new substances (products).
Example : BaCl2 (aq) + H2SO4 (aq) ⟶ BaSO4 (s) + 2HCl (aq)
Give an example of each of the following chemical changes.
(a) A reaction involving
(i) change of state
(ii) formation of a precipitate
(b) An exothermic and an endothermic reaction involving carbon as one of the reactants.
(c) A reaction where colour change is noticed.
Answer
(a) An example of:
(i) A reaction involving change of state — Ammonia gas reacts with hydrogen chloride gas to produce solid ammonium chloride.
NH3 (g) + HCl (g) ⇌ NH4Cl (s)
(ii) A reaction involving formation of a precipitate — Take a solution of silver nitrate in a test tube and add solution of sodium chloride, a curdy white ppt. is formed.
AgNO3 (aq) + NaCl (aq) ⟶ AgCl ↓ + NaNO3 (aq)
(b) Exothermic reaction involving carbon — Combustion of methane gives out large amount of energy.
CH4 + 2O2 ⟶ CO2 ↑ + 2H2O + Heat
Endothermic reaction involving carbon — When carbon is heated with sulphur at high temperature, liquid carbon disulphide is formed.
C + 2S CS2
(c) A reaction where colour change is noticed — When a few pieces of iron are dropped into a blue coloured copper sulphate solution, the blue colour of the solution fades and eventually turns into light green due to the formation of ferrous sulphate.
What is a chemical change? Give two examples of a chemical change?
Answer
A chemical change is a permanent change in which the chemical composition of a substance is changed and one or more new substances with different chemical compositions and different properties are formed.
Examples :
Pb(NO3)2 (s) + 2KI (s) ⟶ 2KNO3 + PbI2 (s)
NaCl (aq.) + AgNO3 (aq.) ⟶ AgCl (white ppt.) ↓ + NaNO3 (aq.)
Define exothermic and endothermic reactions. Give two examples in each case.
Answer
A chemical reaction in which heat is given out is called an exothermic reaction. It causes a rise in temperature.
Examples:
Combustion of methane gives out large amount of energy.
CH4 + 2O2 ⟶ CO2 ↑ + 2H2O + HeatWhen carbon burns in oxygen to form carbon dioxide, lot of heat is produced.
C + O2 ⟶ CO2 ↑ + Heat
A chemical reaction in which heat is absorbed is called an endothermic reaction. It causes a fall in temperature.
Example :
When carbon is heated with sulphur at high temperature, liquid carbon disulphide is formed.
C + 2S CS2Calcium carbonate decomposes to carbon dioxide and calcium oxide when heated to 1000°C
CaCO3 (s) CaO (s) + CO2 (g)
Why is energy involved in a chemical change?
Answer
A chemical reaction involves the breaking up of chemical bonds between atoms resulting in absorption of energy in the form of heat, and simultaneous formation of bonds with release of energy. These two types of energies are different from each other, i.e., there is either a surplus or a deficit of energy during the reaction.
Therefore, in a chemical reaction, energy is involved.
A metal X (used for galvanization) is immersed in the aqueous solution of a blue salt YSO4. Metal Y is deposited on the surface of the solution and a strip of metal X dissolves.
(a) Predict the metal X.
(b) What type of reaction is it?
(c) Write the word equation for this reaction and balance it.
Answer
(a) Zinc (Zn)
(b) Displacement reaction
(c) Copper(II) sulphate + Zinc ⟶ Zinc sulphate + Copper ↓
CuSO4 (aq.) + Zn ⟶ ZnSO4 + Cu↓
Explanation — Galvanization involves coating iron with zinc (Zn) to prevent rusting.
So, Metal X ⟶ Zinc (Zn).
Since Zn displaces Y from its sulphate solution, Zn must be more reactive than Y. The blue salt indicates YSO4 is CuSO4 (copper(II) sulphate), which is blue in color.
So, Metal Y ⟶ Copper (Cu)
YSO4 ⟶ CuSO4
A more reactive metal (zinc) displaces a less reactive metal (copper) from its salt solution.
CuSO4 (aq.) + Zn ⟶ ZnSO4 + Cu↓
State the main characteristics of chemical reactions. Give at least one example in each case.
Answer
The main characteristics of chemical reactions are :
Evolution of gas
Zn(NO3)2 4NO2 + 2ZnO + O2 ↑Change of colour
Change of state
2H2O (l) 2H2 (g) + O2 (g)Formation of precipitate
NaCl (aq.) + AgNO3 (aq.) ⟶ AgCl (white ppt.) ↓ + NaNO3 (aq.)
State the effects of endothermic and exothermic reactions on the surroundings with examples.
Answer
In exothermic reactions heat is given out, hence it causes a rise in temperature.
CH4 + 2O2 ⟶ CO2 ↑ + 2H2O + Heat
In endothermic reactions heat is absorbed, hence it causes a fall in temperature.
C + 2S CS2
Complete and balance the following reactions:
(a) NaCl (aq.) + AgNO3 (aq.) ⟶
(b) Pb(NO3)2 + KI ⟶
(c) CuCO3
(d) Pb(NO3)2
(e) CO2 + H2O
Answer
(a) NaCl (aq.) + AgNO3 (aq.) ⟶ AgCl (white ppt.) ↓ + NaNO3 (aq.)
(b) Pb(NO3)2 (s) + 2KI (s) ⟶ 2KNO3 + PbI2 (s)
(c) CuCO3 (s) CuO (s) + CO2 (g)
(d) Pb(NO3)2 2PbO + 4NO2 + O2
(e) 6CO2 + 6H2O C6H12O6 + 6O2
What do you observe in the following cases?
(a) Lead nitrate is heated.
(b) Silver chloride is exposed to sunlight.
(c) Hydrogen peroxide is exposed to sunlight
(d) H2S gas is passed through copper sulphate solution
(e) Barium chloride is added to sodium sulphate solution.
(f) Water is added to quick lime
(g) Sodium chloride solution is added to silver nitrate solution.
Answer
(a) When white crystalline solid Lead nitrate is heated, it decomposes into a buff yellow residue of lead monoxide. Reddish brown gas Nitrogen dioxide and colourless gas Oxygen are evolved.
(b) When silver chloride (white) is exposed to sunlight, it undergoes photochemical decomposition forming a grey metal, silver and a greenish yellow gas, chlorine.
2AgCl (s) 2Ag (s) + Cl2 (g)
(c) When hydrogen peroxide is exposed to sunlight, it changes to water and colourless, odourless oxygen gas is evolved.
2H2O2 2H2O + O2 ↑
(d) When H2S is passed through copper sulphate solution, a black precipitate of copper sulphide is obtained
CuSO4 + H2S ⟶ CuS [black] + H2SO4
(e) White coloured precipitate of barium sulphate is formed when sodium sulphate is mixed with barium chloride.
Na2SO4 (aq.) + BaCl2 (aq.) ⟶ 2NaCl (aq.) + BaSO4 [white ppt.] ↓
(f) When calcium oxide (quick lime) combines with water, a vigorous reaction takes place with the liberation of a large amount of heat (exothermic reaction) and calcium hydroxide is formed. Heat produced boils the water and a hissing sound is produced.
CaO (s) + H2O (l) ⟶ Ca(OH)2 (s) + Heat
(g) When sodium chloride is added to the silver nitrate solution, a white curdy precipitate of silver chloride is formed.
NaCl (aq.) + AgNO3 (aq.) ⟶ AgCl (white ppt.) ↓ + NaNO3 (aq.)
Discuss the factors that affect a chemical change.
Answer
Certain chemical reactions occur only when electricity is passed through the reactants.
2NaCl 2Na + Cl2 ↑Some chemical reactions occur when the involved substances are subjected to high pressure.
N2 + 3H2 2NH3 ↑Some chemical reactions need a catalyst to accelerate or decelerate the rate at which they occur.
2H2O2 2H2O + O2 ↑Some chemical reactions take place by the action of light.
2AgCl (s) 2Ag (s) + Cl2 (g)Some chemical reactions take place when two substances are mixed in their solid state.
Pb(NO3)2 (s) + 2KI (s) ⟶ 2KNO3 + PbI2 (s)Some chemical reactions occur only on heating
Balance these reactions and state what type of reactions they are:
(a) NaNO3 ⟶ NaNO2 + O2
(b) AgNO3 + Zn ⟶ Zn(NO3)2 + Ag
(c) Fe + HCl ⟶ FeCl2 + H2
(d) AgNO3 ⟶ Ag + NO2 + O2
(e) NaBr + Cl2 ⟶ NaCl + Br2
(f) PbO + C ⟶ Pb + CO2
(g) KClO3 ⟶ KCl + O2
(h) AgNO3 + HCl ⟶ AgCl ↓ + HNO3
(i) NH3 + HCl ⟶ NH4Cl
(j) N2 + H2 ⟶ NH3
Answer
(a) 2NaNO3 ⟶ 2NaNO2 + O2 ↑
Decomposition reaction
(b) 2AgNO3 + Zn ⟶ Zn(NO3)2 + 2Ag
Displacement reaction
(c) Fe + 2HCl ⟶ FeCl2 + H2 ↑
Displacement reaction
(d) 2AgNO3 ⟶ 2Ag + 2NO2 ↑ + O2 ↑
Decomposition reaction
(e) 2NaBr + Cl2 ⟶ 2NaCl + Br2
Displacement reaction
(f) 2PbO + C ⟶ 2Pb + CO2 ↑
Displacement reaction
(g) 2KClO3 ⟶ 2KCl + 3O2 ↑
Decomposition reaction
(h) AgNO3 + HCl ⟶ AgCl ↓ + HNO3
Double decomposition reaction
(i) NH3 + HCl ⟶ NH4Cl
Direct combination reaction
(j) N2 + 3H2 ⟶ 2NH3 ↑
Direct combination reaction
Explain the different types of double decomposition reactions by giving suitable equations.
Answer
Different types of double decomposition reactions are:
Precipitation reaction — A reaction between two compounds in aqueous solution state to give two new compounds one of which is insoluble (precipitate) is called a Precipitation reaction.
For example, when H2S is passed through copper sulphate solution, a black precipitate of copper sulphide is obtained.
CuSO4 (aq) + H2S (g) ⟶ CuS [black ppt.] ↓ + H2SO4Neutralization reaction — The reaction between an acid and a base that forms salt and water only is referred to as Neutralization reaction.
For example, Sodium chloride (base) reacts with HCl (acid) to form salt and water only.
NaOH + HCl ⟶ NaCl + H2O
Explain thermal dissociation and thermal decomposition reactions with examples. What is the difference between them ?
Answer
A reversible decomposition reaction brought about only by heat is called thermal dissociation reaction.
For example, on application of heat, ammonium chloride decomposes into Ammonia and HCl:
NH4Cl ⇌ NH3 + HCl
A chemical reaction in which a compound decomposes to give two new elements / a new compound & an element / two new compounds on application of heat without any recombination on cooling is called a Thermal decomposition reaction.
For example, calcium carbonate decomposes to carbon dioxide and calcium oxide when heated to 1000°C
CaCO3 (s) CaO (s) + CO2 (g)
Difference between the two is that thermal dissociation is a reversible decomposition reaction whereas, thermal decomposition is an irreversible reaction.
Metal X was placed in lead nitrate solution. A thin layer of lead metal deposits on metal X. Which is more reactive metal X or lead ? State the type of reaction and give an example of this type.
Answer
Metal X is more active metal because it displaced the metal lead from lead nitrate. It is a displacement reaction.
CuSO4 + Zn ⟶ ZnSO4 + Cu
Zn is more active metal than Cu, and it displaces Cu from copper sulphate.
What type of reaction is respiration ? Explain.
Answer
Respiration is an exothermic reaction as it involves combustion of glucose with oxygen in the cells of the body releasing heat energy that is used by cells to perform various functions such as muscle contraction, nerve impulse transmission, and protein synthesis. The equation for respiration reaction is:
C6H12O6 + 6O2 ⟶ + 6CO2 ↑ + 6H2O + Heat energy
Discuss some decomposition reactions occurring inside our body.
Answer
Digestion of food in our body is an example of decomposition reaction.
The starch present in the food we eat decomposes into glucose and sugar. Proteins undergo decomposition to form amino acids. Fats and oils are decomposed to fatty acids and finally oxidized by respiration into carbon dioxide and water.
Starch Glucose CO2 + H2O + Energy
K, Na, Ca, Mg, Al, Zn, Fe, Pb, Cu, H, Hg, Ag
Pick an element from the reactivity series given above and write the equations for the the following:
(a) Metal hydroxide on heating forms metal oxide and water vapour.
(b) Metal hydroxide on heating forms metal, oxygen and water vapours.
(c) Metal nitrate decomposes to give two products only.
(d) Metal nitrate on heating forms metal oxide, nitrogen dioxide and oxygen.
(e) Metal nitrate on heating forms metal, nitrogen dioxide and oxygen.
(f) Metal carbonate which is stable to heat.
(g) Metal carbonate which forms metal oxide and carbon dioxide on heating.
(h) The heating effect on bivalent metal hydrogen carbonate.
Answer
(a) Metal hydroxide on heating forms metal oxide and water vapour — Calcium hydroxide
Ca(OH)2 CaO + H2O
(b) Metal hydroxide on heating forms metal, oxygen and water vapours — silver hydroxide
4AgOH 4Ag + O2 + 2H2O
(c) Metal nitrate decomposes to give two products only — potassium nitrate
2KNO3 2KNO2 + O2 ↑
(d) Metal nitrate on heating forms metal oxide, nitrogen dioxide and oxygen — Lead nitrate
2Pb(NO3)2 2PbO + 4NO2 + O2 ↑
(e) Metal nitrate on heating forms metal, nitrogen dioxide and oxygen — Silver nitrate
2AgNO3 2Ag + 2NO2 + O2 ↑
(f) Metal carbonate which is stable to heat — Potassium carbonate
Potassium carbonate is stable to heat
(g) Metal carbonate which forms metal oxide and carbon dioxide on heating — Magnesium carbonate
MgCO3 MgO + CO2 ↑
(h) The heating effect on bivalent metal hydrogen carbonate — Magnesium bicarbonate
Mg(HCO3)2 MgCO3 + H2O + CO2 ↑
KI + Cl2 ⟶ KCl + I2
(a) Balance the equation given above.
(b) Which type of reaction is it ?
(c) Which element is more reactive ?
(d) What will be the product formed if KCl reacts with I2 ?
Answer
(a) 2KI + Cl2 ⟶ 2KCI + I2
(b) Displacement reaction
(c) Chlorine
(d) No reaction, because Iodine being less reactive than chlorine will not be able to displace chlorine from KCl.