Volume of certain amount of a gas at 25°C and 100 cm Hg pressure is 80 mL. The gas is expanded to 160 mL keeping the temperature constant. Calculate the pressure of the expanded gas.
Answer
V1 = Initial volume of the gas = 80 mL
P1 = Initial pressure of the gas = 100 cm Hg
V2 = Final volume of the gas = 160 mL
P2 = Final pressure of the gas = ?
By Boyle's Law:
Substituting the values :
∴ Final pressure of the gas = 50 cm of Hg.
At a particular temperature, a certain quantity of gas occupies a volume of 74 cm3 at a pressure of 760 mm. If the pressure is decreased to 740 mm, what will be the volume of the gas at the same temperature?
Answer
V1 = Initial volume of the gas = 74 cm3
P1 = Initial pressure of the gas = 760 mm
P2 = Final pressure of the gas = 740 mm
V2 = Final volume of the gas = ?
By Boyle's Law:
Substituting the values :
∴ Final volume of the gas = 76 cm3
A student performed an experiment to measure pressure and volume of a gas at constant temperature and noted the following:
| Pressure (mm of Hg) | Volume (cm3) |
|---|---|
| 100 | 80 |
| 125 | x |
| 200 | 40 |
| y | 32 |
Calculate the value of x and y. Which law was used in the calculations? Draw graphs to show:
(i) volume plotted against pressure.
(ii) PV plotted against pressure
Answer
(a) V1 = Initial volume of the gas = 80 cm3
P1 = Initial pressure of the gas = 100 mm of Hg
P2 = Final pressure of the gas = 125 mm of Hg
V2 = Final volume = ?
By Boyle's Law:
Substituting the values :
∴ x = 64 cm3
(b) V1 = Initial volume of the gas = 40 cm3
P1 = Initial pressure of the gas = 200 mm of Hg
V2 = Final volume of the gas = 32 cm3
P2 = Final pressure of the gas = ?
By Boyle's Law:
Substituting the values :
∴ y = 250 mm of Hg
(i) Graph of volume plotted against pressure :
| Pressure (mm of Hg) | Volume (cm3) |
|---|---|
| 100 | 80 |
| 125 | 64 |
| 200 | 40 |
| 250 | 32 |
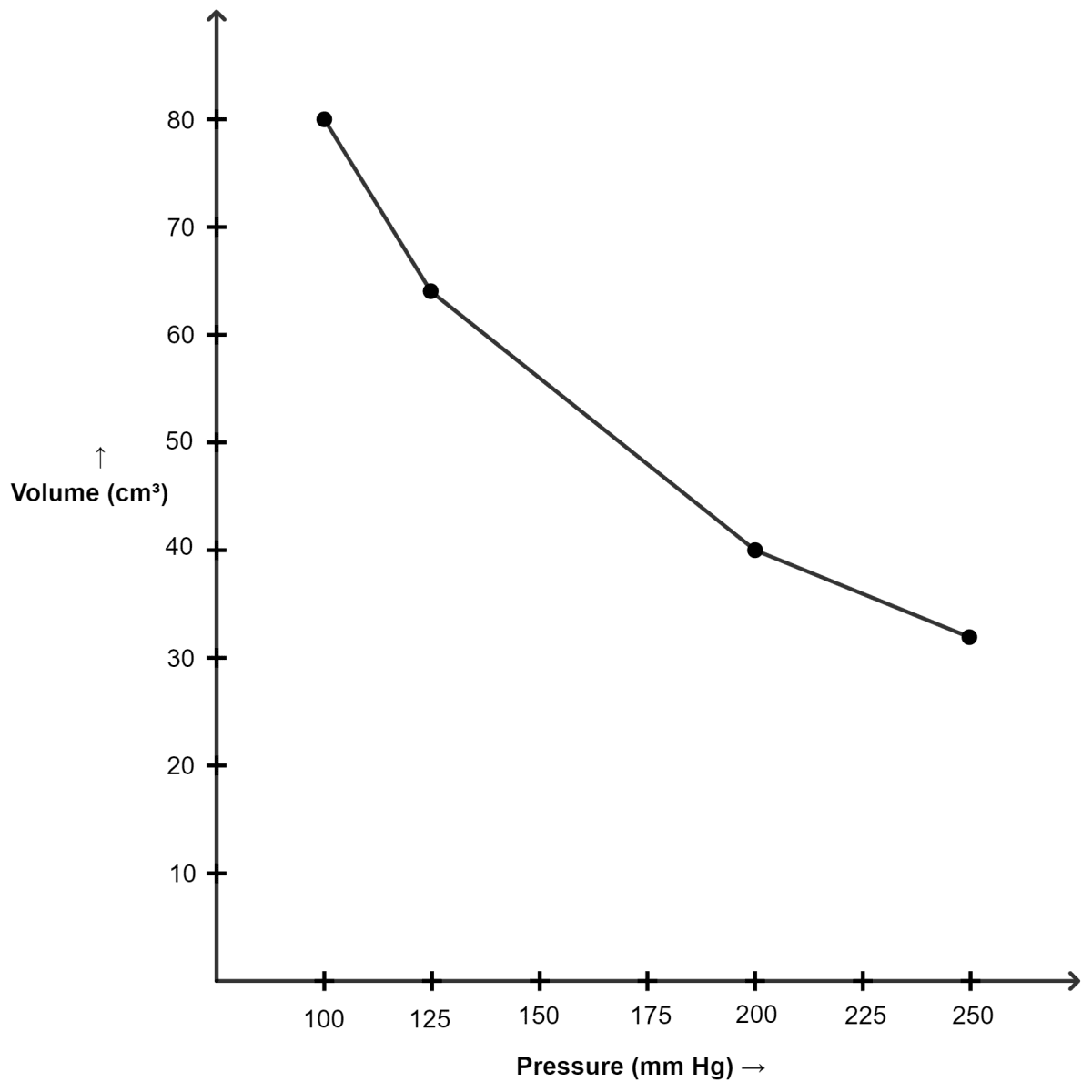
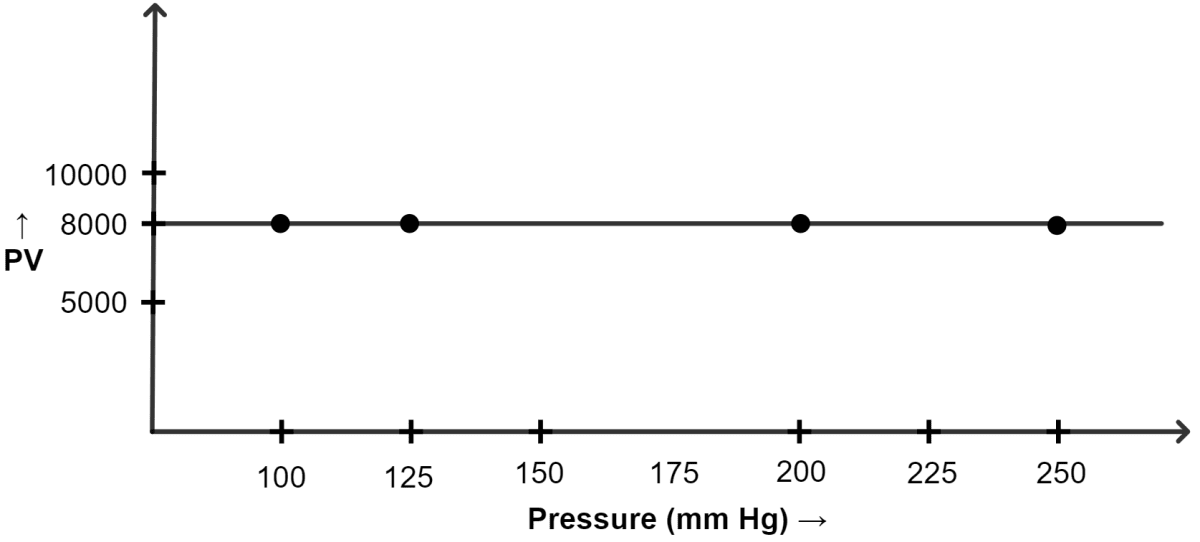
(ii) Graph of PV plotted against pressure :
| Pressure (mm of Hg) | Volume (cm3) | PV |
|---|---|---|
| 100 | 80 | 8000 |
| 125 | 64 | 8000 |
| 200 | 40 | 8000 |
| 250 | 32 | 8000 |
At a constant temperature, volume of a gas was found to be 400 cm3 at a pressure of 760 mm Hg. If the pressure of the gas is increased by 25%, find the new volume.
Answer
V1 = Initial volume of the gas = 400 cm3
P1 = Initial pressure of the gas = 760 mm
P2 = Final pressure of the gas
= increased by 25% of 760 mm Hg
= of 760 mm Hg
= ( x 760) + 760
= 190 + 760 = 950 mm
V2 = Final volume = ?
By Boyle's Law:
Substituting the values :
∴ Final volume = 320 cm3
A vessel of capacity 600 cm3 contains hydrogen gas at a pressure of 330 cm Hg. What will be the pressure of hydrogen gas, when the vessel is connected to another vessel of 300 cm3 capacity ?
Answer
V1 = Initial volume of the gas = 600 cm3
P1 = Initial pressure of the gas = 330 cm Hg
V2 = Final volume = 600 + 300 = 900 cm3
P2 = Final pressure of the gas = ?
By Boyle's Law:
Substituting the values :
∴ Final pressure = 220 cm of Hg
At constant temperature, a gas is at a pressure 1080 mm Hg. If the volume is decreased by 40%, find the new pressure of the gas.
Answer
V1 = Initial volume of the gas = V
P1 = Initial pressure of the gas = 1080 mm of Hg
V2 = Final volume of the gas = decreased by 40%
= 60% of V1 = V = V
P2 = Final pressure of the gas = ?
By Boyle's Law:
Substituting the values :
∴ Final pressure = 1800 mm of Hg
At constant temperature, the pressure of a gas is 940 mm of Hg. Calculate the pressure if the volume of the gas in decreased by 20%.
Answer
V1 = Initial volume of the gas = V
P1 = Initial pressure of the gas = 940 mm of Hg
V2 = Final volume of the gas = decreased by 20%
= 80% of V1 = V = V
P2 = Final pressure of the gas = ?
By Boyle's Law:
Substituting the values :
∴ Final pressure = 1175 mm Hg
If the pressure of 800 cm3 of a gas is tripled what will be the change in the volume?
Answer
V1 = Initial volume of the gas = 800 cm3
P1 = Initial pressure of the gas = P
P2 = Final pressure of the gas = 3P
V2 = Final volume of the gas = ?
By Boyle's Law:
Substituting the values :
∴ Final volume is one-third of initial volume.
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3, if temperature is kept constant?
Answer
Given,
V1 = Initial volume of the gas = 500 dm3
P1 = Initial pressure of the gas = 1 bar.
V2 = Final volume = 200 dm3
P2 = Final pressure of the gas = ?
By Boyle's Law:
Substituting the values :
∴ Final pressure of the gas = 2.5 bar.
Two litres of a gas is enclosed in a vessel at a pressure of 760 mm Hg. If temperature remains constant, calculate pressure when volume changes to 4 dm3.
Answer
V1 = 2 lits.
P1 (Initial pressure) = 760 mm Hg
V2 = 4 dm3
P2 (Final pressure) = ?
By Boyle's Law:
Substituting the values :
∴ Final pressure of the gas = 380 mm Hg.
Convert the following:
(i) 37 K to °C
(ii) 273 K to °C
(iii) -27°C to K
(iv) 27°C to K
Answer
(i) °C = Kelvin - 273 = 37 - 273 = -236°C
(ii) °C = Kelvin - 273 = 273 - 273 = 0°C
(iii) Kelvin = °C + 273 = -27 + 273 = 246 K
(iv) Kelvin = °C + 273 = 27 + 273 = 300 K
20 mL of hydrogen gas at -13°C is heated to 117°C at constant pressure. Find the new volume of hydrogen.
Answer
V1 = Initial volume of the gas = 20 mL
T1 = Initial temperature of the gas = -13°C = -13 + 273 = 260 K
T2 = Final temperature of the gas = 117°C = 117 + 273 = 390 K
V2 = Final volume of the gas =?
By Charles' Law:
Substituting the values :
∴ Final volume = 30 mL
At what temperature in degree centigrade will the volume of a gas, which is originally at 0°C, double itself, pressure remaining constant?
Answer
V1 = Initial volume of the gas = V
T1 = Initial temperature of the gas = 0°C = 273 K
V2 = Final volume of the gas = 2V
T2 = Final temperature of the gas = ?
By Charles' Law:
Substituting the values :
∴ Final temperature in °C = 546 - 273 = 273°C
Calculate the volume (in cm3) of air expelled from a vessel containing 0.4 litres of it at 250 K, when it is heated to 27°C at the same pressure.
Answer
1 lit = 1000 cm3
∴ 0.4 lit = 400 cm3
V1 = Initial volume of the gas = 400 cm3
T1 = Initial temperature of the gas = 250 K
T2 = Final temperature of the gas = 27°C = 27 + 273 = 300 K
V2 = Final volume of the gas = ?
By Charles' Law:
Substituting the values :
Volume of air expelled = 480 - 400 = 80 cm3
What will be the volume of a gas when 3 litres of it is cooled down from 27°C to -73°C at constant pressure?
Answer
V1 = Initial volume of the gas = 3 litres
T1 = Initial temperature of the gas = 27°C = 27 + 273 = 300 K
T2 = Final temperature of the gas = -73°C = -73 + 273 = 200 K
V2 = Final volume of the gas = ?
By Charles' Law:
Substituting the values :
∴ Final volume = 2 litres.
To what temperature must a gas at 300 K be cooled down in order to reduce its volume to rd of its original volume, pressure remaining constant?
Answer
V1 = Initial volume of the gas = V
T1 = Initial temperature of the gas = 300 K
V2 = Final volume of the gas = V
T2 = Final temperature of the gas = ?
By Charles' Law:
Substituting the values :
∴ Final temperature = 100 K
Prove that the volume of a gas at 273°C is twice its volume at 273 K, at constant pressure.
Answer
V1 = Initial volume of the gas = V
T1 = Initial temperature of the gas = 273 K
T2 = Final temperature of the gas = 273°C = 273 + 273 = 546 K
V2 = Final volume of the gas = ?
By Charles' Law:
Substituting the values :
∴ Volume of a gas at 273°C is twice its volume at 273 K
A gas occupies 3 litres at 0°C. What volume will it occupy at -20°C pressure remaining constant?
Answer
V1 = Initial volume of the gas = 3 litres
T1 = Initial temperature of the gas = 0°C = 273 K
T2 = Final temperature of the gas = -20°C = -20 + 273 = 253 K
V2 = Final volume of the gas = ?
By Charles' Law:
Substituting the values :
∴ Final volume = 2.78 litres.
A gas at 240 K is heated to 127°C. Find the percentage change in the volume of the gas (pressure remaining constant).
Answer
V1 = Initial volume of the gas = V cc
T1 = Initial temperature of the gas = 240 K
T2 = Final temperature of the gas = 127°C = 127 + 273 = 400 K
V2 = Final volume of the gas = ?
By Charles' Law:
Substituting the values :
Increase in vol. = - V =
Percentage increase in vol. = x 100 = x 100 = 66.67%
∴ Percentage increase in the volume of the gas = 66.67%
Certain amount of a gas occupies a volume of 0.4 litre at 17°C. Calculate the temperature at which the volume of gas is (a) doubled (b) reduced to half, pressure remaining constant?
Answer
(a) To find the temperature so that volume gets doubled :
V1 = Initial volume of the gas = 0.4 litre
T1 = Initial temperature of the gas = 17°C = 17 + 273 = 290 K
V2 = Final volume of the gas = double of initial = 2 x 0.4 = 0.8 litre
T2 = Final temperature of the gas = ?
By Charles' Law:
Substituting the values :
∴ Final temperature in °C = 580 - 273 = 307°C
(b) To find the temperature so that volume gets reduced to half :
V1 = Initial volume of the gas = 0.4 litre
T1 = Initial temperature of the gas = 17°C = 17 + 273 = 290 K
V2 = Final volume of the gas = reduced to half of initial = 0.2 litre
T2 = Final temperature of the gas = ?
By Charles' Law:
Substituting the values :
∴ Final temperature in °C = 145 - 273 = -128°C
The graph of PV vs P for a gas is :
- parabolic
- hyperbolic
- a straight line parallel to X-axis
- a straight line passing through origin
Answer
a straight line parallel to X-axis
Reason — The graph of PV vs P is a straight line parallel to the pressure axis.
The absolute temperature value that corresponds to 27°C is :
- 200 K
- 300 K
- 400 K
- 246 K
Answer
300 K
Reason — Celsius values can be converted to Kelvin values by adding 273 to degree celsius values. Hence, 27°C + 273 = 300 K
Volume-temperature relationship is given by :
- Boyle
- Gay Lussac
- Dalton
- Charles
Answer
Charles
Reason — Charles' law states that volume of a given mass of a dry gas is directly proportional to its absolute (Kelvin) temperature, if the pressure remains constant.
If pressure is doubled for a fixed mass of a gas, its volume will become :
- 4 times
- 1⁄2 times
- 2 times
- No change
Answer
1⁄2 times
Reason — According to Boyle's law volume of a given mass of a dry gas is inversely proportional to its pressure at constant temperature.
Hence, if pressure is doubled for a fixed mass of a gas, its volume will become 1⁄2 times.
The S.I. unit of temperature is :
- Pascal
- °C
- Kelvin
- °F
Answer
Kelvin
Reason — The S.I. unit of temperature is Kelvin.
Which of the following temperatures is known as the steam point ?
- 273 K
- 373 K
- 290 K
- 273°C
Answer
373 K
Reason — The steam point on Celsius scale is 100°C and corresponding to that steam point on Kelvin scale is 273 + 100 = 373 K.
The graph shown below gives the statement for :
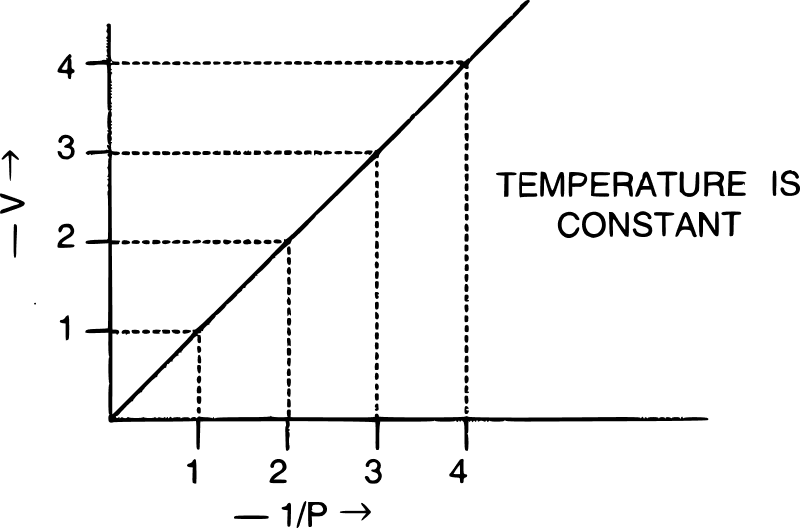
- Henry's law
- Charles' law
- Boyle's law
- Daltons' law
Answer
Boyle's law
Reason — For graphical verification of Boyle's law a graph of V vs 1/P is a straight line passing through the origin.
'The volume of a given amount of a gas is directly proportional to its absolute temperature at a constant pressure' is the statement of :
- Gay-Lussac's law
- Boyle's law
- Charles' law
- Mendeleev's law
Answer
Charles' law
Reason — Charles' law states that volume of a given mass of a dry gas is directly proportional to its absolute (Kelvin) temperature, if the pressure remains constant.
The volume of a given mass of a gas at constant temperature and 10 atmospheric pressure is 10 litres. Its volume at 5 atmospheres will be :
- 5 litres
- 10 litres
- 15 litres
- 20 litres
Answer
20 litres
Reason — Given,
V1 = 10 l
P1 = 10 atm
P2 = 5 atm
V2 = ?
According to Boyle's law, P1V1 = P2V2
So, 10 x 10 = 5 x V2
V2 = = 20 l
STP is called standard temperature and pressure. The standard temperature and standard pressure respectively are :
- 273 K and 760 mm
- 0°C and 760 cm
- 273°C and 1 atmosphere
- 373 K and 76 cm
Answer
273 K and 760 mm
Reason — The standard values chosen are 0°C or 273 K for temperature and 1 atm or 760 mm of Hg for pressure. These values are known as standard temperature and pressure (STP).
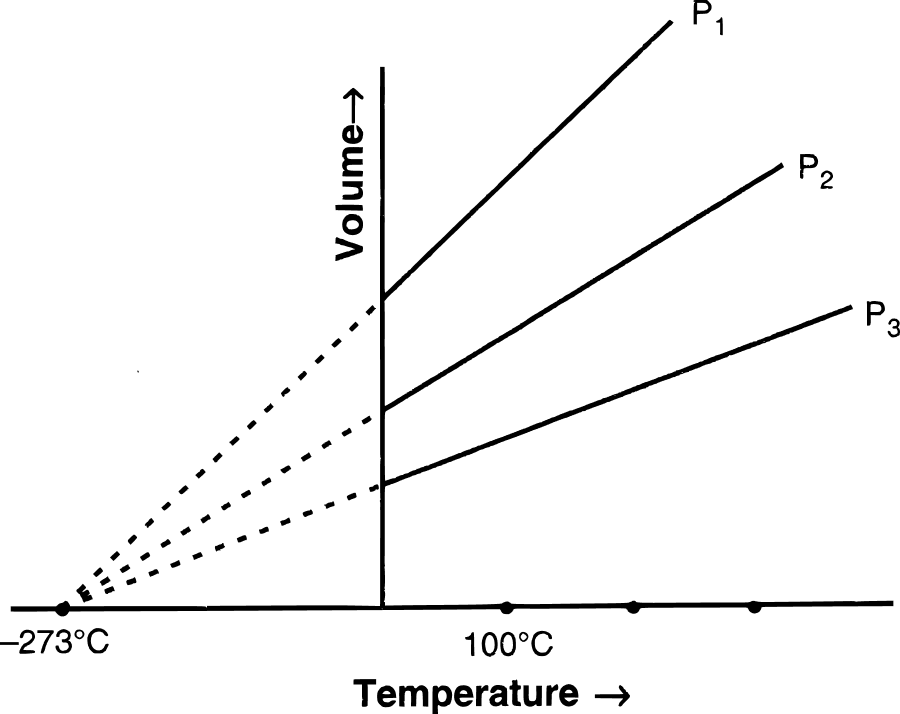
Following figures illustrate graphical representations of gas laws. the figure which represents Charles' law is/are :
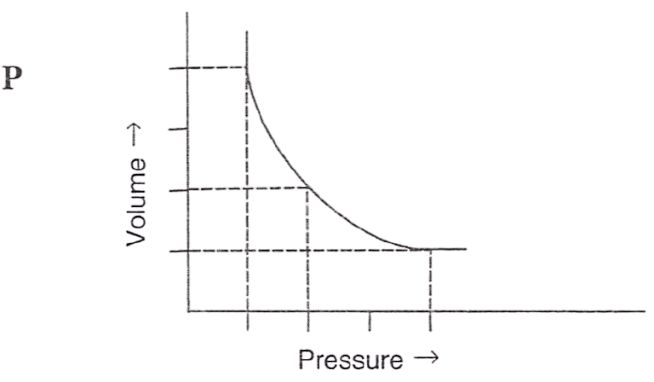
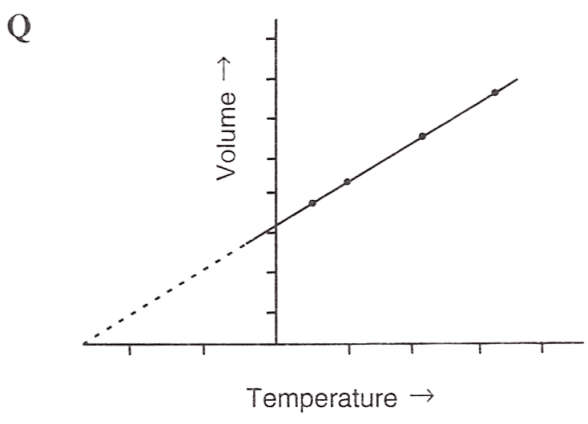
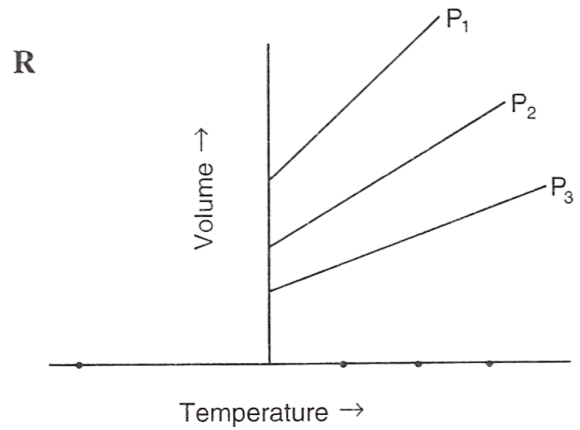
- Only P
- Only Q
- Only R
- Both Q and R
Answer
Both Q and R
Reason — In graph Q and R, the relationship between volume and temperature of gas is plotted at constant pressure. The straight line proves that volume is directly proportional to temperature (at constant pressure) as per Charles' law.
The term used for the graph of volume vs pressure for the verification of Boyle's law is :
P — Isobar
Q — Isotherm
R — Isotope
- Only P
- Only Q
- Only R
- Both P and Q
Answer
Only Q
Reason — Boyle's law states that volume of a given mass of a dry gas is inversely proportional to its pressure at constant temperature. Hence, the term isoterm, meaning at constant temperature is used to describe the graphs.
Assertion (A): Inflating a balloon seems to violate Boyle's law.
Reason (R): Boyle's law is valid for a fixed mass. Here, the mass of gas is changing.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— When air is blown into a balloon, volume and pressure inside the balloon increase. Here, Boyle's law is not violated as the law is valid for a definite mass, whereas mass increases when more air is blown into the balloon. Hence, both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): The volume occupied by a gas is negligible at zero degree centigrade temperature.
Reason (R): Molecular motion ceases at 0 K.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is false but R is true.
Explanation— Gases follow charles's law, volume of given mass of gas is directly proportional to its absolute temperature. At 0°C, a gas still behaves mostly like an ideal gas under standard pressure, and its volume is not negligible. Hence, the assertion (A) is false.
Molecular motion increases with rising temp and decreases with lowering temperature. When the temperature is zero on kelvin scale, the molecular motion ceases. Hence, the reason (R) is true.
Assertion (A): Gases exert pressure on the walls of the container they are kept in.
Reason (R): Gas molecules have a large kinetic energy. They strike the walls of the container in which they are kept with a certain force. The force per unit area is responsible for the pressure of the gas.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— Gas exerts uniform pressure on the walls of the containing vessel. The reason is that the particles (molecules) of the gas collide with each other and with the walls of the containing vessel. Since a large number of particles (molecules) suffer collisions with the wall, an appreciable force acts on the wall. The force exerted on a unit area of the wall of the vessel is equal to the pressure of the gas. Hence, both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): The volumes of gases are first converted into STP conditions and then compared.
Reason (R): Volume does not vary with temperature and pressure in gases.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation— Gases are often compared at Standard Temperature and Pressure (STP) to have a common reference point. Hence, converting volumes to STP (typically 0°C and 1 atm) is a standard procedure. Hence, the assertion (A) is true.
For a given mass of a gas, a change in one or more than one variable, i.e., pressure, volume and temperature, results in a change in the remaining variables. Hence, the reason (R) is false.
Assertion (A): When stating the volume of a gas, the pressure and temperature should also be given.
Reason (R): The volume of a gas is inversely proportional to temperature and directly proportional to pressure.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false
Explanation— The change in one or more than one variable, i.e., pressure, volume and temperature, results in a change in the remaining variables. So, when stating the volume of a gas, the pressure and temperature should also be given. Hence, the assertion (A) is true.
The volume of a gas is directly proportional to temperature and inversly proportional to pressure. Hence, the reason (R) is false.
Assertion (A): Gases diffuse easily and form homogeneous mixtures.
Reason (R): Molecules of other substances occupy the intermolecular spaces present in gases.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— Gases have a natural tendency to mix with one another i.e., they diffuse easily and form homogeneous mixtures. Hence, the assertion (A) is true.
Inter-particle (inter-molecular) spaces in a gas are very large. When two gases are brought in contact with each other, their molecules mix with each other in such a manner that a homogeneous gaseous mixture is formed. Hence, the reason (R) is true and it is the correct explanation of assertion (A).
Assertion (A): At zero Kelvin (-273°C), the volume occupied by a gas is considered as zero.
Reason (R): Theoretically, molecular motion completely ceases at zero Kelvin.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— According to Charles’s Law, gas volume decreases linearly with temperature (in Kelvin). So, theoretically at 0 K, the volume of an ideal gas would be zero. Hence, the assertion (A) is true. As temperature increases, molecular motion increases, and when temperature decreases, molecular motion also decreases. Theoretically, this suggests that when the temperature is zero, molecular motion ceases. Hence, the reason (R) is true and it is the correct explanation of assertion (A).
Assertion (A): For a given amount of any gas, the product PV is always constant.
Reason (R): Pressure is inversely proportional to volume at constant temperature.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— The product of pressure and volume of a given mass of a gas is constant if the temperature remains constant. This can be deduced by platting values of P against values of PV at a constant temperature. Hence, the assertion (A) is true. Volume of a given mass of a dry gas is inversly proportional to its pressure at constant temperature. Hence, the reason (R) is true.
Reason (R) states boyle's law, which explains 'the product of volume and pressure of a given masss of a dry gas at constant temperature is constant. Hence, reason (R) is the correct explanation of assertion (A).
Assertion (A): It is necessary to mention conditions while stating the volumes of gases.
Reason (R): The volume of a gas does not depend on any condition.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation— For a given mass of a gas, a change in one or more than one variable, i.e., pressure, volume and temperature, results in a change in the remaining variables. So it is necessary to mention conditions while stating the volumes of gases. Hence, the assertion (A) is true. Volume of gas depends on changing pressure and temperature. Volume and temperature relate directly whereas, volume and pressure relate inversely. Hence, The reason (R) is false.
Match the following:
| Column A | Column A |
|---|---|
| (a) Cm3 | (i) Pressure |
| (b) Kelvin | (ii) PV = P1V1 |
| (c) Torr | (iii) Volume |
| (d) Boyle’s law | (iv) = |
| (e) Charles law | (v) = |
| (vi) temperature |
Answer
| Column A | Column A |
|---|---|
| (a) Cm3 | (iii) Volume |
| (b) Kelvin | (vi) temperature |
| (c) Torr | (i) Pressure |
| (d) Boyle’s law | PV = P1V1 |
| (e) Charles law | (iv) = |
Fill in the blanks:
(a) The average kinetic energy of the molecules of a gas is proportional to the ...............
(b) The temperature on the Kelvin scale at which molecular motion completely ceases is called ...............
(c) If the temperature is reduced to half, ............... would also reduce to half.
(d) The melting point of ice is ............... Kelvin.
Answer
(a) The average kinetic energy of the molecules of a gas is proportional to the absolute temperature.
(b) The temperature on the Kelvin scale at which molecular motion completely ceases is called absolute zero.
(c) If the temperature is reduced to half, volume would also reduce to half.
(d) The melting point of ice is 273 Kelvin.
Correct the following statements, wherever necessary.
(a) The volume of a gas is inversely proportional to its pressure at constant temperature.
(b) Volume of a fixed mass of a gas is directly proportional to its temperature, pressure remaining constant.
(c) 0°C is equal to zero Kelvin.
(d) Standard temperature is 25°C.
(e) The boiling point of water is 273 K.
Answer
(a) The volume of a given mass of a dry gas is inversely proportional to its pressure at constant temperature.
(b) Volume of a fixed mass of a dry gas is directly proportional to its absolute temperature (Kelvin), pressure remaining constant.
(c) 0°C is equal to 273 Kelvin
(d) Standard temperature is 0°C or 273 K.
(e) The boiling point of water is 373 K.
State (i) the three variables for gas laws (ii) S.I. units of these variables.
Answer
(i) The three variables for gas laws are Volume (V), Pressure (P), Temperature (T)
(ii) S.I. units of these variables:
Volume: Cubic metre (m3)
Pressure: Pascal (Pa)
Temperature: Kelvin (K)
Write the value of:
(a) Standard temperature in:
(i) °C (ii) K
(b) Standard pressure in:
(i) atm (ii) mm Hg (iii) cm Hg (iv) torr
Answer
(a) Standard temperature
(i) 0°C (ii) 273 K
(b) Standard pressure
(i) 1 atmospheric pressure (atm)
(ii) 760 mm Hg
(iii) 76 cm of Hg
(iv) 760 torr
State the following:
(a) Volume of a gas at 0 Kelvin.
(b) Absolute temperature of a gas at 7°C.
(c) Gas equation.
(d) Ice point in absolute temperature.
(e) S.T.P. conditions
Answer
(a) Volume of a gas at 0 Kelvin is zero.
(b) Absolute temperature of a gas at 7°C is 7 + 273 = 280 K.
(c) The gas equation is
=
(d) Ice point in absolute temperature = 0 + 273 = 273 K
(e) Standard temperature = 0°C = 273 K
Standard pressure = 760 mm Hg = 76 cm Hg = 1 atmosphere (atm)
What do you understand by gas?
Answer
Gas is a state of matter in which inter-particle attraction is weak and inter-particle space is so large that the particles become completely free to move randomly in the entire available space.
What is diffusion ? Give an example to illustrate it.
Answer
Diffusion is the process of gradual mixing of two substances, kept in contact, by molecular motion.
If a jar of chlorine is opened in one end of the room, the odour of the gas can be detected in the entire room, because its particles start moving in a haphazard manner in all directions, till there is an equal concentration throughout the room.
How is molecular motion related with temperature?
Answer
Molecular motion is directly proportional to temperature. When temperature increases molecular motion increases and when temperature decreases molecular motion decreases.
Define absolute zero and absolute scale of temperature.
Answer
The temperature on the Kelvin scale at which molecular motion completely ceases is called absolute zero. Absolute zero is -273°C.
The temperature scale with its zero at -273.15°C and whose each degree is equal to one degree on the Celsius scale, is called the Kelvin or absolute scale of temperature.
(a) Define S.T.P.
(b) Why is it necessary to compare gases at S.T.P.?
Answer
(a) The standard values chosen are 0°C or 273 K for temperature, and 1 atmospheric unit (atm) or 760 mm Hg for pressure. These standard values are known as standard temperature and pressure (S.T.P.).
(b) Since volume of a gas changes remarkably with change in temperature and pressure, it becomes necessary to choose standard values of temperature and pressure to which gas volumes can be referred.
State the laws which are represented by the following graphs.
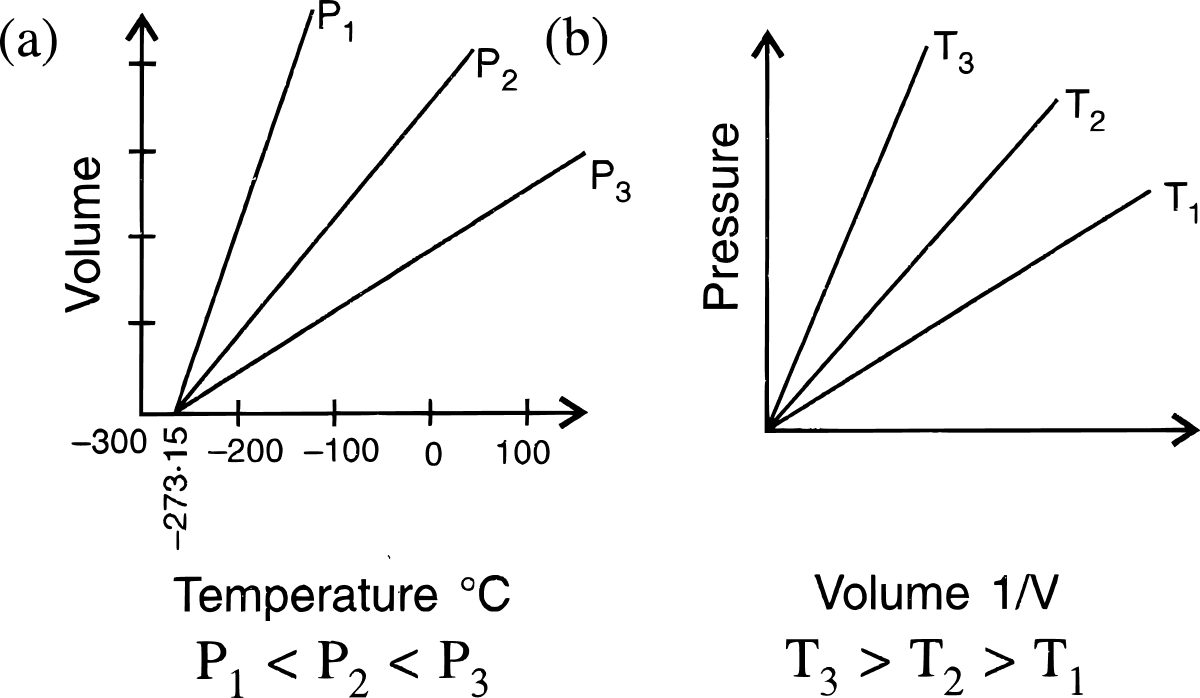
Answer
(a) Charles' law
(b) Boyle's law
What is the relationship between the Celsius and the Kelvin scales of temperature?
Answer
The size of 1 degree on the Kelvin scale is the same as the size of 1 degree on the Celsius scale i.e., unit size on Kelvin scale is equal to the unit size on Celsius scale.
The value on the Celsius scale can be converted to Kelvin scale by adding 273 to it. Kelvin scale values can be converted to degree Celsius values by subtracting 273 from it.
The molecular theory states that the pressure exerted by a gas in closed vessel results from the gas molecules striking against the walls of the vessel. How will the pressure change if :
(a) the temperature is doubled keeping the volume constant
(b) the volume is made half of its original value keeping the temperature constant?
Answer
(a) This can be explained on the basis of Gas equation
=
When volume is constant we get,
=
Hence, when temperature is doubled, pressure is doubled, as pressure and temperature are directly proportional.
(b) This can be explained on the basis of Boyle's law
P1V1 = P2V2
Hence, when the volume is made half of its original value keeping the temperature constant, pressure becomes double, as pressure and volume are inversely proportional.
(a) What is the need for the Kelvin scale of temperature?
(b) What is the boiling point of water on the Kelvin scale? Convert it into centigrade scale.
Answer
(a) Kelvin scale makes application and use of gas laws simple. More significantly, all values on Kelvin scale are positive.
(b) Boiling point of water on the Kelvin scale is 373 K.
Temperature in °C = K - 273
So, 373 K - 273 = 100°C.
Give reasons for the following :
(a) All temperatures in the absolute (Kelvin) scale are in positive figures.
(b) Gases have lower density compared to that of solids or liquids.
(c) Gases exert pressure in all directions.
(d) It is necessary to specify the pressure and temperature of a gas while stating its volume.
(e) Inflating a balloon seems to violate Boyle's law.
(f) Mountaineers carry oxygen cylinders with them.
(g) Gas fills completely the vessel in which it is kept.
Answer
(a) The temperature -273°C is called absolute zero and it is theoretically the lowest temperature that can ever be achieved. The absolute (Kelvin) scale has its zero at -273°C. As temperatures lower than -273°C are not possible, hence, all temperatures in Kelvin scale are in positive figures.
(b) The number of molecules per unit volume in a gas is very small as compared to solids and liquids. Gases have large inter-molecular spaces between their molecules. Therefore gases have lower density compared to that of solids or liquids.
(c) The moving particles of a gas collide with each other and also with the walls of the container. Due to these collisions gas molecules exert pressure. It has been found that at a given temperature, time and area, the same number of molecules of a gas strike against the walls of the container. Thus, gases exert the same pressure in all directions.
(d) Since volume of a gas changes remarkably with change in temperature and pressure, it becomes necessary to choose standard values of temperature and pressure to which gas volumes can be referred.
(e) Boyle's law states that the volume of a dry gas is inversely proportional to its pressure at a constant temperature. When we inflate a balloon, the volume and pressure of the air increase simultaneously, seemingly violating Boyle's law. However, this is not the case because according to the law, the mass of the gas should remain constant. In the balloon scenario, as more air is blown into the balloon, the mass of the gas increases, ensuring Boyle's law is not violated.
(f) Atmospheric pressure is low at high altitudes, so air is less dense. Hence, lesser quantity of oxygen is available for breathing. Thus, mountaineers have to carry oxygen cylinders with them.
(g) Gas is a state of matter in which inter-particle attraction is weak and inter-particle space is so large that the particles become completely free to move randomly in the entire available space. Hence, it fills completely the vessel in which it is kept.
Give the assumptions of the kinetic molecular theory.
Answer
The assumptions of the kinetic molecular theory are :
- Composition of gases — Gases are made of tiny particles moving in all possible directions at all possible speeds. The molecules are negligibly small as compared to the volume of the occupied gas.
- Gases have neither a fixed volume nor a fixed shape — There is negligible force of attraction between the particles (gas molecules). Therefore, the particles (gas molecules) are free to move in the entire space available to them. Their movement is only restricted by the container, hence it takes the shape of the container.
- Gases exert pressure in all directions — The moving particles of gas collide with each other and with the walls of the container. Due to these collisions, gas molecules exert pressure. It has been found, that at a given temperature, time and area, the same number of molecules of a gas strike against the walls of the container. Thus, gases exert the same pressure in all directions.
- Gases are highly compressible — There are large inter-particle (inter-molecular) space between gas molecules, and this accounts for high compressibility of gases.
- Gases are highly expansible — Gases increase in volume with a decrease in pressure and an increase in temperature.
- Gases have low density — The number of molecules per unit volume in a gas is very small due to the large intermolecular spaces between their molecules. Therefore, gases have very low density.
- Gases have a natural tendency for diffusion — Due to large intermolecular spaces, the molecules of two gases mix with each other in such a manner that a homogeneous gaseous mixture is formed.
- Gases can be liquefied — On cooling, the kinetic energy of the molecules of a gas is reduced and on applying pressure on a cooled gas, the molecules come closer. Thus, the gas liquefies.
Explain Boyle's law on the basis of the kinetic theory of matter.
Answer
According to the kinetic theory of gases, the number of particles and their average kinetic energy in a given mass of gas remain constant. When the volume of a gas is halved, the particles have less space to move, resulting in double the number of molecules striking the container walls per unit area and thus doubling the pressure. Conversely, doubling the volume gives the particles more space, reducing the number of molecules striking the walls per unit area by half and halving the pressure. This demonstrates Boyle's law: as pressure increases, the volume of a gas decreases at a constant temperature.
Explain Charles' law on the basis of the kinetic theory of matter.
Answer
According to the kinetic theory of gases, the average kinetic energy of gas particles is directly proportional to the absolute temperature. Increasing the temperature causes the particles to move faster and strike the container walls more frequently and forcefully. When pressure is constant, the volume of the gas increases proportionally. Therefore, at constant pressure, the volume of a gas is directly proportional to its temperature (Charles' law).
How did Charles' law lead to the concept of an absolute scale of temperature?
Answer
According to Charles' law, when pressure (P) is constant,
∴ Volume at -273°C = = 0
Hence, the volume of a gas would be reduced to zero at -273°C. This temperature is called absolute zero.
This allowed the scientists to establish an absolute temperature scale (known as Kelvin scale) by defining absolute zero as the point where volume of a gas would theoretically be zero.
(a) State Boyle's Law.
(b) Give its
(i) mathematical expression,
(ii) graphical representation and
(iii) significance.
Answer
(a) Boyle's law states that the volume of a given mass of a dry gas is inversely proportional to its pressure at a constant temperature.
(b) (i) Mathematical expression of Boyle's law :
Suppose a gas occupies volume V1 when its pressure is P1; then
V1 α or
V1 = or
P1V1 = k = constant
If V2 is the volume occupied when the pressure is P2 at the same temperature, then
V2 α or
V2 = or
P2V2 = k = constant
∴ P1V1 = P2V2 = k; at constant temperature.
(ii) Graphical representation of Boyle's Law:
(a) V vs : a straight line passing through the origin is obtained.

(b) V vs P : a hyperbolic curve in the first quadrant is obtained.
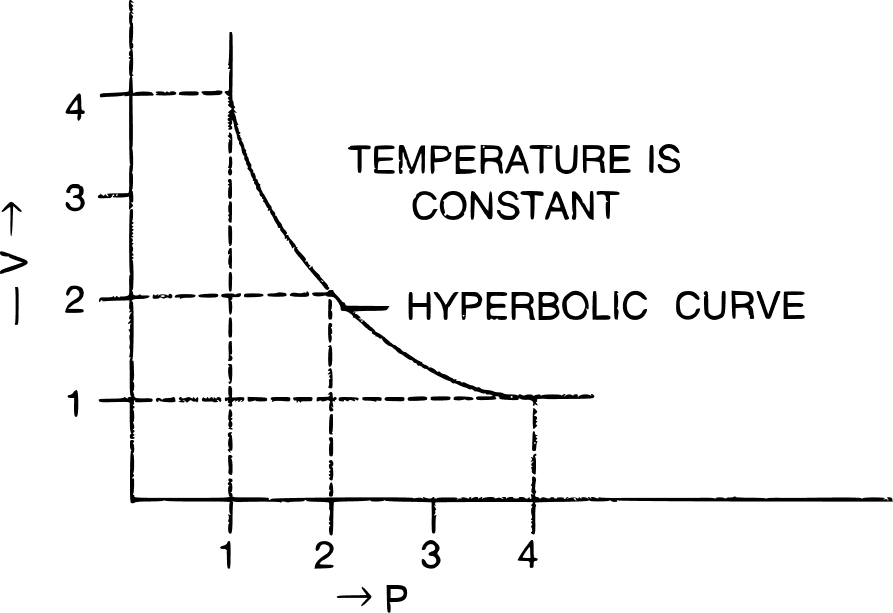
(c) PV vs P : a straight line is obtained parallel to the pressure axis.
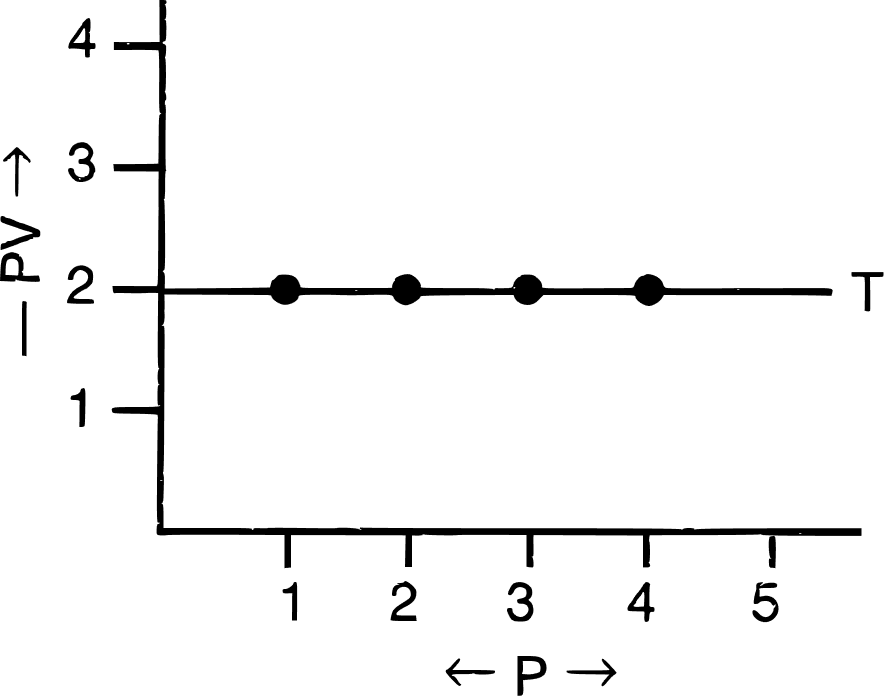
(iii) Significance of Boyle's law :
On increasing pressure, volume decreases. The gas becomes denser. Thus, at constant temperature, the density of a gas is directly proportional to pressure.
(a) State Charles' law.
(b) Give its
(i) Graphical representation,
(ii) mathematical expression and
(iii) Significance.
Answer
(a) Charles law states that pressure remaining constant, the volume of a given mass of dry gas increases or decreases by of its volume at 0°C for each 1°C increase or decrease in temperature, respectively.
(b) (i) Graphical representation of Charles' law : The relationship between the volume and the temperature of a gas can be plotted on a graph. A straight line is obtained.
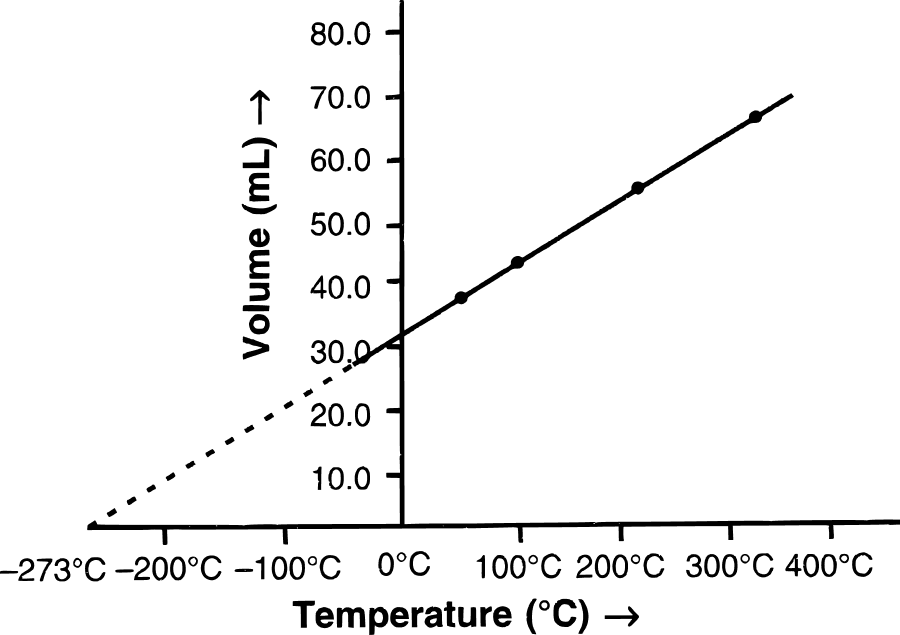
(ii) Mathematical expression of Charles' law :
Suppose, a gas occupies V1 cm3 at T1 temperature and V2 cm3 at T2 temperature, then by Charles' law:
V1 α T1
or V1 = kT1 (k is constant)
or = k and
V2 α T2
or = k
∴ = = k (at constant pressure)
(iii) Significance of Charles' Law :
Volume of a given mass of a gas is directly proportional to its temperature, hence, density decreases with an increase in temperature.
What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?
Answer
When the gas is collected over water, it is moist and contains water vapour. The total pressure exerted by moist gas is equal to the sum of the partial pressure of the dry gas and the pressure exerted by water vapour. The partial pressure of water vapour is called as aqueous tension.
Ptotal = Pgas + Pwater vapour
Pgas = Ptotal – Pwater vapour
Actual pressure of gas = Total pressure – Aqueous tension
Thus, to obtain the actual pressure, the pressure which is due to moisture in the gas has to be subtracted.
During the chemistry practical when hydrogen sulphide gas having offensive odour is prepared for some test in the laboratory, we can smell the gas from even 50 metres away. Explain the phenomenon.
Answer
The phenomenon is Diffusion.
Diffusion is the process of gradual mixing of two substances, kept in contact, by molecular motion.
The molecules of hydrogen sulphide gas collide with air particles and due to the collisions of the particles, they start moving in a haphazard manner in all possible directions. Due to this, the molecules of hydrogen sulphide gas quickly spread in the surroundings and we can smell the gas from even 50 metres away.
(a) State the law verified by the following figure:
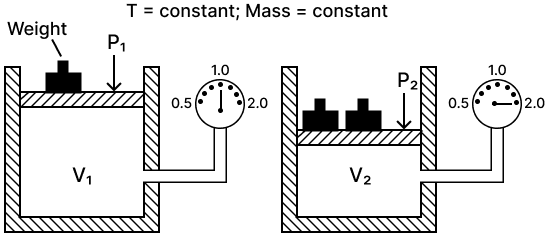
(b) Draw P.V. isothermal for the above law.
Answer
(a) Boyle's law — It states that the volume of a given mass of a dry gas is inversely proportional to its pressure at a constant temperature i.e., P1V1 = P2V2
(b) P.V. isothermal for Boyle's law is shown below :

(a) State the law which the following graph verifies.

(b) Derive the mathematical expression for it.
(c) Give one application/use where the above law is employed.
Answer
(a) Charles' law — It states that pressure remaining constant, the volume of a given mass of dry gas increases or decreases by of its volume at 0°C for each 1°C increase or decrease in temperature, respectively.
(b) Let V0 be the volume of a fixed mass of a gas at 0°C and let V be its volume at temperature t°C at constant pressure. Then according to Charles' law,
V = Vo + t (when P is constant)
V = Vo (1 + )
= Vo ()
V = T where T = 273 + t
For a given mass of a gas,
= constant
∴ V = k x T (where k is constant)
or V α T and = K
Suppose a gas occupies V1 cm3 at T1 temperature and V2 cm3 at T2 temperature, then by Charles law,
V1 α T1
or V1 = kT1 (k is constant)
or = k and
V2 α T2
or = k
∴ = = k (at constant pressure)
(c) Volume of a given mass of a gas is directly proportional to its temperature, hence, density decreases with an increase in temperature. This is the reason that hot air is filled into balloons used for meteorological purposes.
Plot V versus absolute T (K) by the given data:
| Temperature | Volume in litres |
|---|---|
| 27°C | 4.8 |
| 77°C | 5.6 |
| 127°C | 6.4 |
| 177°C | 7.2 |
| 227°C | 8.0 |
(a) The graph between V and T is a ............... .
(b) Check whether the line passes through the origin.
(c) Which law is obeyed ?
Answer
Converting temperature to Kelvin Scale :
| Temp (°C) | Temp (K) | Volume (litres) |
|---|---|---|
| 27 | 300 | 4.8 |
| 77 | 350 | 5.6 |
| 127 | 400 | 6.4 |
| 177 | 450 | 7.2 |
| 227 | 500 | 8.0 |
Graph of V versus absolute T is shown below :
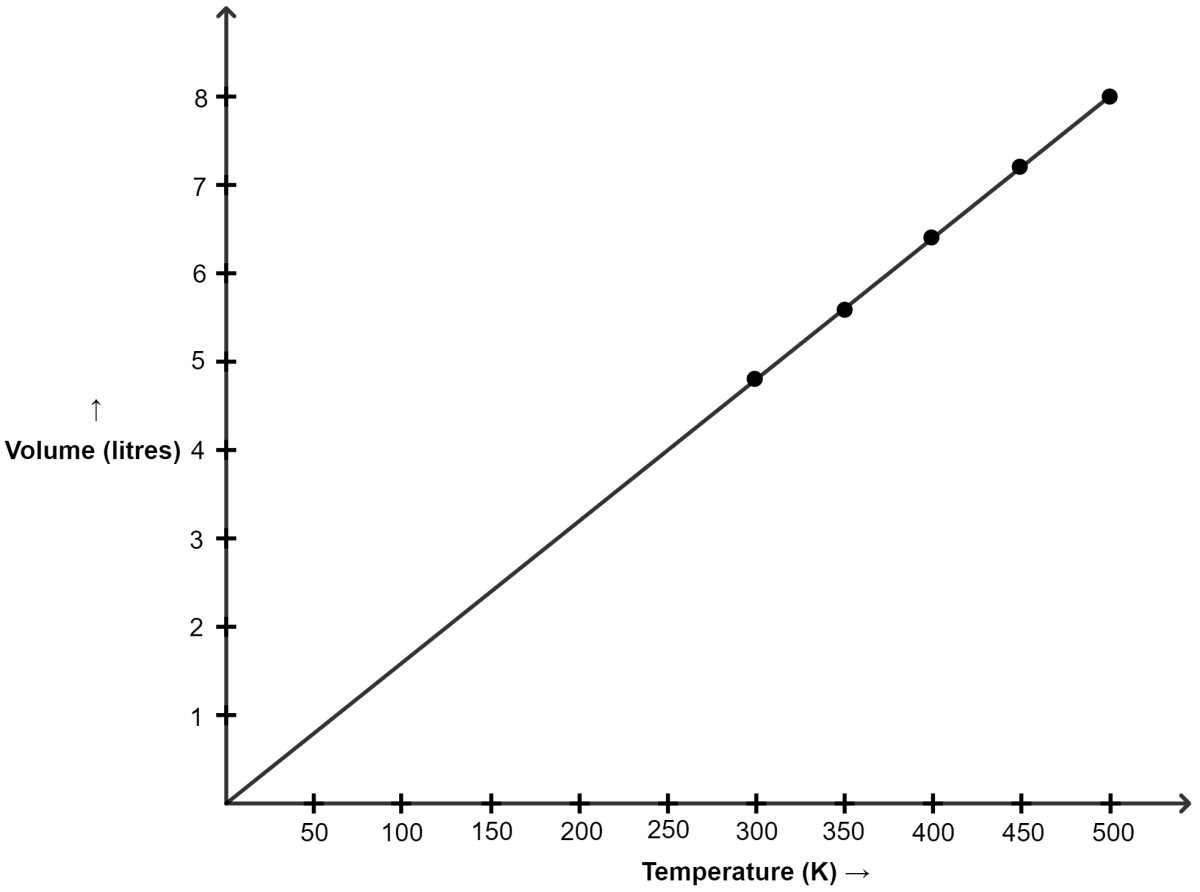
(a) The graph between V and T is a straight line.
(b) Yes, as we can see in the graph, the line passes through the origin.
(c) Charles' law
Convert :
(i) 273°C to kelvin
(ii) 293 K to °C
Answer
(i) Kelvin = °C + 273 = 273 + 273 = 546 K
(ii) °C = Kelvin - 273 = 293 - 273 = 20°C
800 cm3 of gas is collected at 654 mm pressure. At what pressure would the volume of the gas reduce by 40% of its original volume, temperature remaining constant?
Answer
V1 = 800 cm3
P1 (Initial pressure) = 654 mm of Hg
V2 = reduces by 40% of it's initial value
= 800 - ( x 800)
= 800 - 320 = 480 cm3
P2 (Final pressure) = ?
By Boyle's Law:
Substituting the values :
∴ Final pressure of the gas = 1090 mm of Hg.
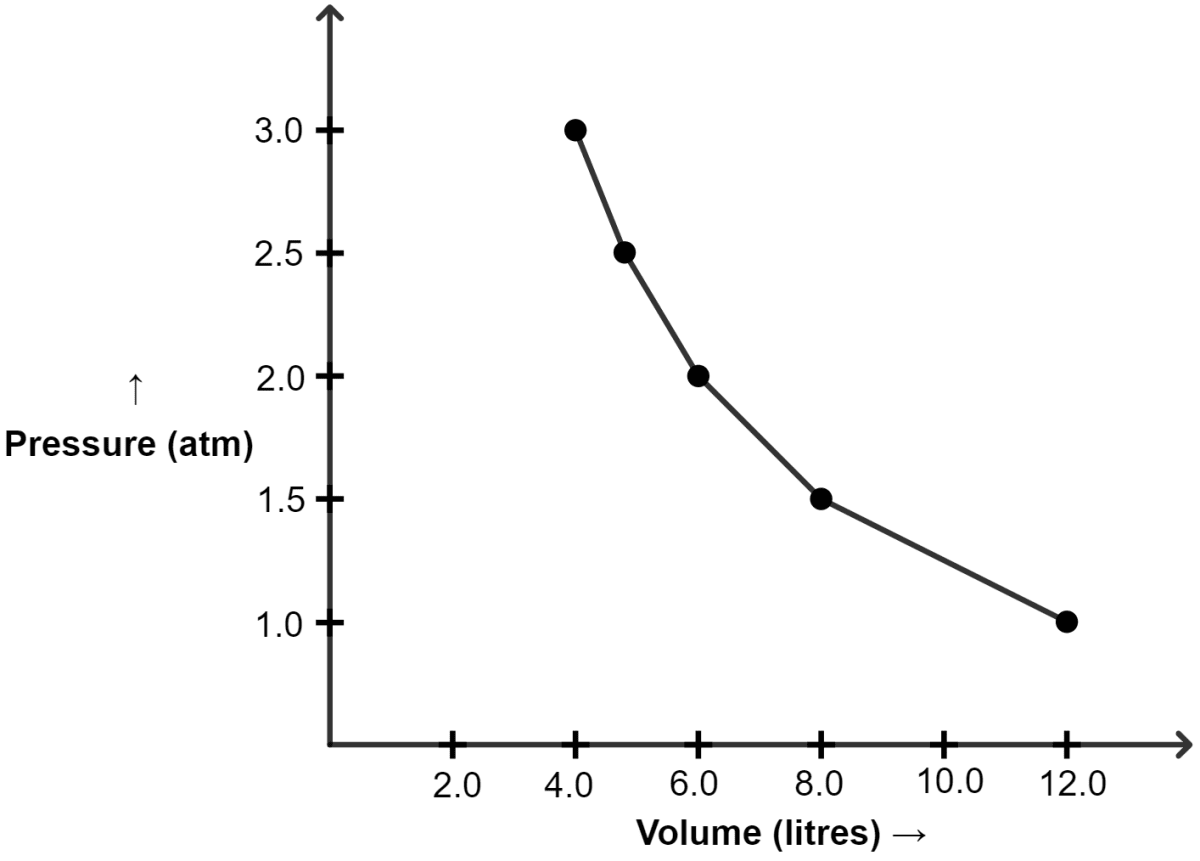
At constant temperature, the effect of change of pressure on volume of a gas was as given below :
| Pressure in atmospheres | Volume in litres |
|---|---|
| 1.0 | 12 |
| 1.5 | 8.0 |
| 2.0 | 6.0 |
| 2.5 | 4.8 |
| 3.0 | 4.0 |
(a) Plot the following graphs :
(i) P vs V
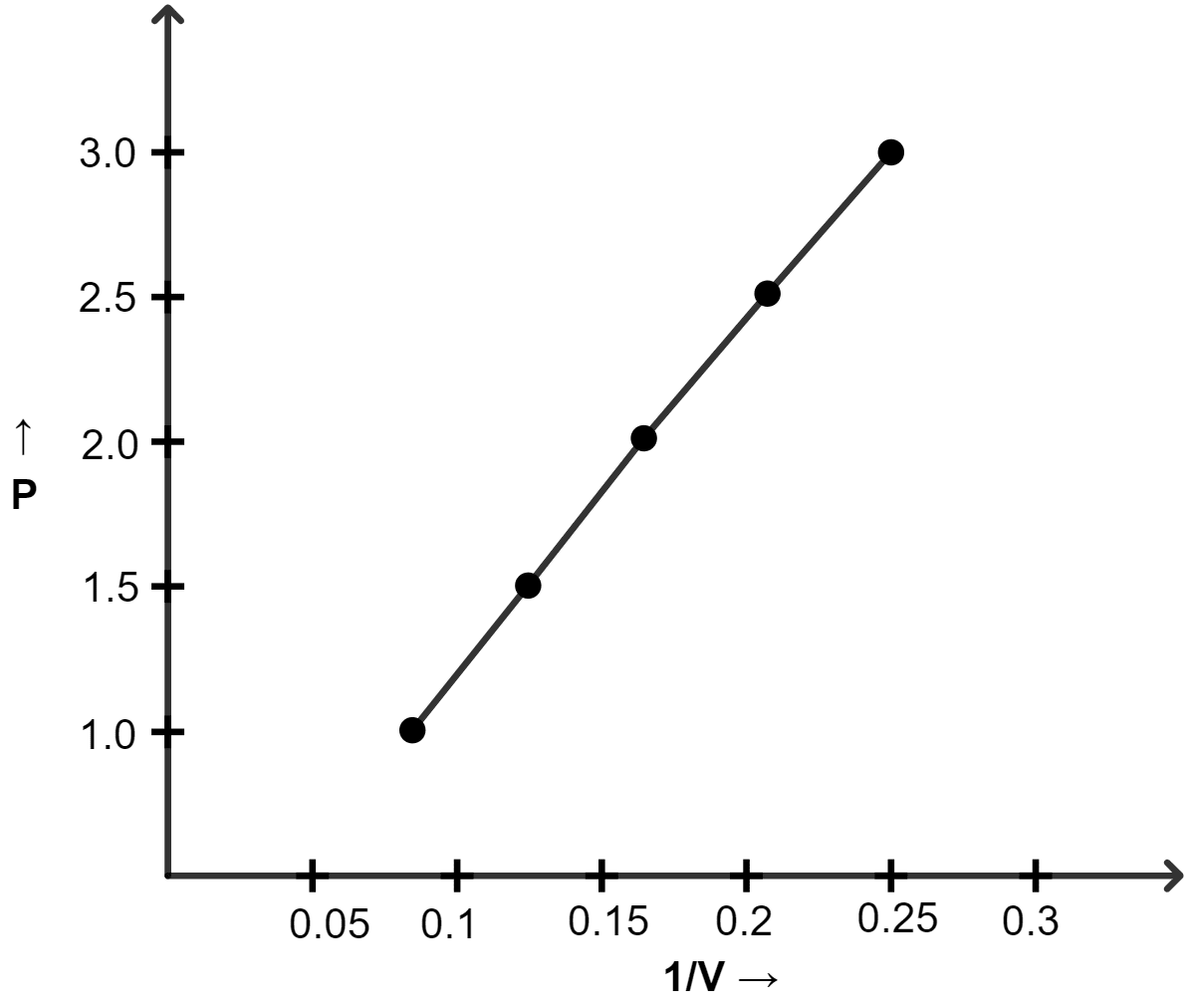
(ii) P vs 1/V
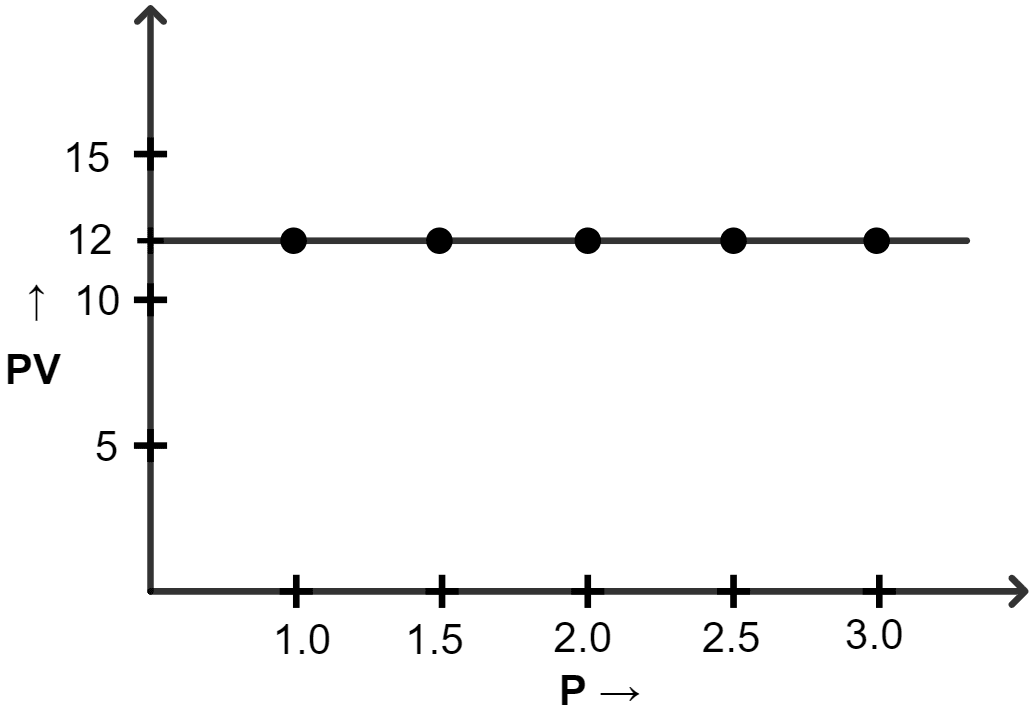
(iii) PV vs P
(b) Assuming that the pressure values given above are correct, find the correct measurement of the volume at 2.3 atmosphere pressure.
Answer
(a)
(i) Graph of P vs V :
| Pressure (atm) | Volume (lit) |
|---|---|
| 1.0 | 12.0 |
| 1.5 | 8.0 |
| 2.0 | 6.0 |
| 2.5 | 4.8 |
| 3.0 | 4.0 |
(ii) Graph of P vs 1/V :
| Pressure (atm) | Volume (lit) | 1/V |
|---|---|---|
| 1.0 | 12 | 0.083 |
| 1.5 | 8.0 | 0.125 |
| 2.0 | 6.0 | 0.166 |
| 2.5 | 4.8 | 0.208 |
| 3.0 | 4.0 | 0.25 |
(iii) Graph of PV vs P :
| Pressure (atm) | Volume (lit) | PV |
|---|---|---|
| 1.0 | 12 | 12 |
| 1.5 | 8.0 | 12 |
| 2.0 | 6.0 | 12 |
| 2.5 | 4.8 | 12 |
| 3.0 | 4.0 | 12 |
(b) By Boyle's Law:
P1V1 = 12 is a constant, hence, V2 = ? at P2 = 2.3 atm
Substituting the values :
Hence, volume = 5.217 lit
A cylinder of 20 litres capacity contains a gas at 100 atmospheric pressure. How many flasks of 200 cm3 capacity can be filled from it at 1 atmosphere pressure temperature remaining constant?
Answer
V1 = 20 lits.
1 lit. = 1000 cm3
∴ 20 lit = 20,000 cm3
P1 (Initial pressure) = 100 atmos.
P2 (Final pressure) = 1 atmos.
V2 = ?
By Boyle's Law:
Substituting the values :
200 cm3 = capacity of 1 flask
∴ Number of flasks required for 20,00,000 cm3 of gas
Hence, number of flask that can be filled = 10,000.
A steel cylinder of internal volume 20 litres is filled with hydrogen at 29 atmospheric pressure. If hydrogen is used to fill a balloon at 1.25 atmospheric pressure at the same temperature, what volume will the gas occupy ?
Answer
V1 = 20 litres
P1 = 29 atmospheric pressure
P2 = 1.25 atmospheric pressure
V2 = ?
By Boyle's Law:
Substituting the values :
∴ Volume occupied = 464 litres.
561 dm3 of a gas at S.T.P. is filled in a 748 dm3 container. If temperature is constant, calculate the percentage change in pressure required.
Answer
V1 = 561 dm3
P1 = P
V2 = 748 dm3
P2 = ?
By Boyle's Law:
Substituting the values :
Change in pressure = P - 0.75P = 0.25P decrease
Percentage decrease in pressure = x 100 = x 100 = 25%
∴ Percentage decrease in the pressure of the gas = 25%
88 cm3 of nitrogen is at a pressure of 770 mm mercury. If the pressure is raised to 880 mm Hg, find by how much the volume will diminish, temperature remains constant.
Answer
V1 = 88 cm3
P1 = 770 mm
P2 = 880 mm Hg
V2 = ?
By Boyle's Law:
Substituting the values :
V2 = 77 cm3
∴ Decrease in volume = 88 - 77 = 11 cm3
A given mass of a gas occupies 143 cm3 at 27°C and 700 mm Hg pressure. What will be its volume at 300 K and 280 mm Hg pressure?
Answer
| Initial conditions | Final conditions |
|---|---|
| P1 = Initial pressure of the gas = 700 mm Hg | P2 = Final pressure of the gas = 280 mm Hg |
| V1 = Initial volume of the gas = 143 cm3 | V2 = Final volume of the gas = ? |
| T1 = Initial temperature of the gas = 27°C = 27 + 273 = 300 K | T2 = Final temperature of the gas = 300 K |
By Gas Law:
Substituting the values :
∴ Final volume of the gas = 357.5 cm3
A gas occupies 500 cm3 at S.T.P. At what temperature will the volume of the gas be reduced by 20% of its original volume, pressure being constant?
Answer
V1 = Initial volume of the gas = 500 cm3
T1 = Initial temperature of the gas = 273 K
V2 = Final volume of the gas = reduced by 20% of its original = 500 - ( x 500) = 500 - 100 = 400 cm3
T2 = Final temperature of the gas = ?
By Charles' Law:
Substituting the values :
Final temperature = 218.4 K
Calculate the final volume of a gas 'X', if the original pressure of the gas, at S.T.P. is doubled and its temperature is increased three times.
Answer
| Initial conditions [S.T.P.] | Final conditions |
|---|---|
| P1 = Initial pressure of the gas = 760 mm Hg | P2 = Final pressure of the gas = (2 x 760) mm Hg |
| V1 = Initial volume of the gas = V | V2 = Final volume of the gas = ? |
| T1 = Initial temperature of the gas = 273 K | T2 = Final temperature of the gas = (3 x 273) K |
By Gas Law:
Substituting the values :
∴ Final volume of the gas = times the original volume.
A sample of carbon dioxide occupies 30 cm3 at 15°C and 740 mm pressure. Find its volume at S.T.P.
Answer
| Initial conditions | Final conditions [S.T.P.] |
|---|---|
| P1 = Initial pressure of the gas = 740 mm Hg | P2 = Final pressure of the gas = 760 mm Hg |
| V1 = Initial volume of the gas = 30 cm3 | V2 = Final volume of the gas = ? |
| T1 = Initial temperature of the gas = 15°C = 15 + 273 = 288 K | T2 = Final temperature of the gas = 273 K |
By Gas Law:
Substituting the values :
∴ Volume of carbon dioxide = 27.7 cm3
What temperature would be necessary to double the volume of a gas, initially at S.T.P., if the pressure is decreased to 50% ?
Answer
| Initial conditions [S.T.P.] | Final conditions |
|---|---|
| P1 = Initial pressure of the gas = 1 atm | P2 = Final pressure of the gas = 0.5 atm |
| V1 = Initial volume of the gas = V | V2 = Final volume of the gas = 2V |
| T1 = Initial temperature of the gas = 273 K | T2 = Final temperature of the gas = ? |
By Gas Law:
Substituting the values :
∴ Final temperature of the gas = 273 K
At 0°C and 760 mm Hg pressure, a gas occupies volume of 100 cm3. Kelvin temperature of the gas is increased by one-fifth and the pressure is increased one and a half times. Calculate the final volume of the gas.
Answer
Initial conditions [S.T.P.] :
P1 = Initial pressure of the gas = 760 mm Hg
V1 = Initial volume of the gas = 100 cm3
T1 = Initial temperature of the gas = 0°C = 273 K
Final conditions :
P2 (Final pressure) = increased one and a half times of P1
= (1 + ) of 760
= x 760
= 3 x 380
= 1140 mm Hg
V2 (Final volume) = ?
T2 (Final temperature) = increased by one-fifth of 273 K
= of 273
= x 273 =
By Gas Law:
Substituting the values :
∴ Final volume of the gas = 80 cm3
It is found, on heating a gas, its volume increases by 50% and pressure decreases to 60% of its original value. If the original temperature was -15°C, find the temperature to which it was heated?
Answer
Initial conditions [S.T.P.] :
P1 = Initial pressure of the gas = P
V1 = Initial volume of the gas = V
T1 = Initial temperature of the gas = -15°C = -15 + 273 = 258 K
Final conditions :
P2 (Final pressure) = pressure decreases to 60% of its original value
= of P
= P
V2 (Final volume) = volume increases by 50% of its original value
= 1 + of V
= of V
= of V
= V
T2 (Final temperature) = ?
By Gas Law:
Substituting the values :
∴ Final temperature of the gas = 232.2 - 273 = -40.8°C
A certain mass of a gas occupies 2 Litres at 27°C and 100 pascal. Find the temperature when volume and pressure become half of their initial values.
Answer
Initial conditions [S.T.P.] :
P1 = Initial pressure of the gas = 100 Pascal
V1 = Initial volume of the gas = 2 Litres
T1 = Initial temperature of the gas = 27°C = 27 + 273 = 300 K
Final conditions :
P2 (Final pressure) = 50 Pascal
V2 (Final volume) = 1 litre
T2 (Final temperature) = ?
By Gas Law:
Substituting the values :
∴ Final temperature of the gas = 75 - 273 = -198°C
2500 cm3 of hydrogen is taken at S.T.P. The pressure of this gas is further increased by two and and a half times (temperature remaining constant). What volume will hydrogen occupy now?
Answer
V1 = 2500 cm3
P1 = 760 mm Hg
P2 = increased by two and a half times
= (1 + ) times of 760 mm Hg
= (1 + ) times of 760 mm Hg
= ( x 760) mm Hg
V2 = ?
By Boyle's Law:
Substituting the values :
∴ Final volume = cm3
Taking the volume of hydrogen as calculated in Q.16 what change must be made in Kelvin (absolute) temperature to return the volume to 2500 cm3 (pressure remaining constant).
Answer
V1 = Initial volume of the gas = cm3
T1 = Initial temperature of the gas = 273 K
V2 = Final volume of the gas = 2500 cm3
T2 = Final temperature of the gas = ?
By Charles' Law:
Substituting the values :
∴ Final temperature = 3.5 times of Kelvin temperature.
A given amount of gas A is confined in a chamber of constant volume. When the chamber is immersed in a bath of melting ice, the pressure of the gas is 100 cm Hg.
(a) What is the temperature, when the pressure is 10 cm Hg?
(b) What will be the pressure, when the chamber is brought to 100°C?
Answer
(a) P1 = Initial pressure of the gas = 100 cm Hg
T1 = Initial temperature of the gas = 273 K
P2 = Final pressure of the gas = 10 cm Hg
T2 = Final temperature of the gas = ?
V1 = V2 = V [constant volume]
By Gas Law:
Substituting the values :
∴ When the pressure is 10 cm Hg, the temperature is 27.3 K.
(b) P1 = Initial pressure of the gas = 100 cm Hg
T1 = Initial temperature of the gas (ice point) = 273 K
P2 = Final pressure of the gas = ?
T2 = Final temperature of the gas = 100°C = 273 + 100 = 373 K
V1 = V2 = V [constant volume]
By Gas Law:
Substituting the values :
∴ When the chamber is brought to 100°C, the pressure is 136.63 cm Hg.
A gas is to be filled from a tank of capacity 10,000 litres into cylinders each having capacity of 10 litres. The condition of the gas in the tank is as follows:
(a) pressure inside the tank is 800 mm of Hg.
(b) temperature inside the tank is -3°C.
When the cylinder is filled, the pressure gauge reads 400 mm of Hg and the temperature is 270 K. Find the number of cylinders required to fill the gas.
Answer
Initial conditions :
P1 = Initial pressure of the gas = 800 mm of Hg
V1 = Initial volume of the gas = 10,000 litres
T1 = Initial temperature of the gas = -3°C = -3 + 273 = 270 K
Final conditions:
P2 (Final pressure) = 400 mm of Hg
V2 (Final volume) = 10 litres x n cylinders
T2 (Final temperature) = 270 K
where n is the number of cylinders = ?
By Gas Law:
Substituting the values :
∴ Number of cylinders = 2000.
A sealed tube (able to withstand a pressure of 3 atmospheres) is filled with air at 27°C and one atmosphere pressure. Find the temperature above which it will burst.
Answer
P1 = Initial pressure of the gas = 1 atm
T1 = Initial temperature of the gas = 27°C = 27 + 273 = 300 K
P2 = Final pressure of the gas = 3 atm
T2 = Final temperature of the gas = ?
V1 = V2 = V [constant volume]
By Gas Law:
Substituting the values :
∴ The temperature above which the tube will burst is 627°C.
Calculate the temperature at which the volume of a gas is doubled if its pressure at the same time increases from 70 cm of Hg to 80 cm of Hg.
Answer
Initial conditions :
P1 = Initial pressure of the gas = 70 cm of Hg
V1 = Initial volume of the gas = V
T1 = Initial temperature of the gas = 273 K
Final conditions:
P2 (Final pressure) = 80 cm of Hg
V2 (Final volume) = 2V
T2 (Final temperature) = ?
By Gas Law:
Substituting the values :
∴ Final temperature of the gas = 624 - 273 = 351°C
A certain gas at -15°C is heated. Its volume increases by 50% and pressure decreases to 75% of its original reading. Calculate the temperature to which it was heated.
Answer
Initial conditions [S.T.P.] :
P1 = Initial pressure of the gas = P
V1 = Initial volume of the gas = V
T1 = Initial temperature of the gas = -15°C = -15 + 273 = 258 K
Final conditions :
P2 (Final pressure) = pressure decreases to 75% of its original value
V2 (Final volume) = Volume increases by 50% of its original value
T2 (Final temperature) = ?
By Gas Law:
Substituting the values :
Solve for T2:
∴ Final temperature of the gas = 290.25 - 273 = 17.25°C
Calculate the volume occupied by 2 g of hydrogen at 27°C and 4 atmosphere pressure, if at S.T.P. it occupies 22.4 litres.
Answer
Initial conditions [S.T.P.] :
P1 = Initial pressure of the gas = 1 atm
V1 = Initial volume of the gas = 22.4 l
T1 = Initial temperature of the gas = 273 K
Final conditions :
P2 (Final pressure) = 4 atm
T2 (Final temperature) = 27°C = 27 + 273 = 300 K
V2 (Final volume) = ?
By Gas Law:
Substituting the values :
∴ Volume occupied = 6.15 l
Which will have greater volume when the following gases are compared at S.T.P.
(a) 1.2 lit N2 at 25°C and 748 mm Hg
(b) 1.25 lit O2 at S.T.P.?
Answer
1.2 lit N2 at 25°C and 748 mm Hg
Initial conditions :
P1 = Initial pressure of the gas = 748 mm Hg
V1 = Initial volume of the gas = 1.2 lit
T1 = Initial temperature of the gas = 25°C = 25 + 273 = 298 K
Final conditions :
P2 (Final pressure) = 760 mm of Hg
T2 (Final temperature) = 273 K
V2 (Final volume) = ?
By Gas Law:
Substituting the values :
∴ Volume of N2 at S.T.P. = 1.081 lit and volume of O2 at S.T.P. is 1.25 lit. Hence, volume of O2 at S.T.P. is greater than N2 at S.T.P.
50 cm3 of hydrogen is collected over water at 17°C and 750 mm Hg pressure. Calculate the volume of dry gas at S.T.P. The water vapour pressure at 17°C is 14 mm Hg.
Answer
Initial conditions :
P1 = 750 - water vapour pressure = 750 - 14 = 736 mm Hg
V1 = Initial volume of the gas = 50 cm3
T1 = Initial temperature of the gas = 17°C = 17 + 273 = 290 K
Final conditions :
P2 (Final pressure) = 760 mm of Hg
T2 (Final temperature) = 273 K
V2 (Final volume) = ?
By Gas Law:
Substituting the values :
∴ Volume of dry gas = 45.6 cm3
(a) Calculate the volume of S.T.P. of 380 cm3 of carbon dioxide at 91°C and 72 mm of Hg pressure.
(b) What will be its volume at 273 K and 70 cm of Hg pressure ?
Answer
(a)
| Initial conditions | Final conditions |
|---|---|
| P1 = Initial pressure of the gas = 72 mm Hg | P2 = Final pressure of the gas = 760 mm Hg |
| V1 = Initial volume of the gas = 380 cm3 | V2 = Final volume of the gas = ? |
| T1 = Initial temperature of the gas = 91°C = 91 + 273 = 364 K | T2 = Final temperature of the gas = 273 K |
By Gas Law:
Substituting the values :
(b)
| Initial conditions | Final conditions |
|---|---|
| P1 = Initial pressure of the gas = 72 mm of Hg | P2 = Final pressure of the gas = 70 cm Hg = 700 mm of Hg |
| V1 = Initial volume of the gas = 380 cm3 | V2 = Final volume of the gas = ? |
| T1 = Initial temperature of the gas = 91°C = 91 + 273 = 364 K | T2 = Final temperature of the gas = 273 K |
By Gas Law:
Substituting the values :
Calculate the volume of dry air at S.T.P. that occupies 28 cm3 at 14°C and 750 mm Hg pressure when saturated with water vapour. The vapour pressure of water at 14°C is 12 mm Hg.
Answer
Initial conditions :
P1 = 750 - water vapour pressure = 750 - 12 = 738 mm Hg
V1 = Initial volume of the gas = 28 cm3
T1 = Initial temperature of the gas = 14°C + 273 = 287 K
Final conditions [S.T.P.] :
P2 (Final pressure) = 760 mm of Hg
T2 (Final temperature) = 273 K
V2 (Final volume) = ?
By Gas Law:
Substituting the values :
∴ Volume of dry gas = 25.9 cm3
L.P.G. cylinder can withstand a pressure of 14.9 atmosphere. The pressure gauge of the cylinder indicates 12 atmosphere at 27°C. Due to a sudden fire in the building the temperature rises. At what temperature will the cylinder explode?
Answer
P1 = Initial pressure of the gas = 12 atm
T1 = Initial temperature of the gas = 27°C = 27 + 273 = 300 K
P2 = Final pressure of the gas = 14.9 atm
T2 = Final temperature of the gas = ?
V1 = V2 = V [constant volume]
By Gas Law:
Substituting the values :
∴ The temperature at which the cylinder will explode = 99.5°C.
22.4 litres of a gas weighs 70 g at S.T.P. Calculate the weight of the gas if it occupies a volume of 20 litres at 27°C and 700 mm Hg of pressure.
Answer
Initial conditions :
P1 = 700 mm Hg
V1 = Initial volume of the gas = 20 litres
T1 = Initial temperature of the gas = 27°C + 273 = 300 K
Final conditions [S.T.P.] :
P2 (Final pressure) = 760 mm of Hg
T2 (Final temperature) = 273 K
V2 (Final volume) = ?
By Gas Law:
Substituting the values :
22.4 l of gas at S.T.P. weighs 70 g
∴ 16.76 l of gas at S.T.P. will weigh
= = 52.38 g
∴ Weight of gas = 52.38 g