Justify the position of Hydrogen in the periodic table.
Answer
Hydrogen is the first element of the periodic table. Its atomic number is 1, and it has only one electron in its valence shell. Therefore, it belongs to the first group and the first period of the periodic table.
As hydrogen shows a dual nature, it resembles alkali metals of Group IA and the halogens of Group VII A (17). Thomson suggested a separate position for hydrogen. He puts hydrogen at the top of the periodic table, that does not disturb the symmetry of the periodic table.
Why does hydrogen show dual nature?
Answer
Hydrogen resembles the alkali metals of Group IA and the halogens of Group VIIA. This is due to the fact that it has only one electron in its valence shell, hence it behaves like the alkali metals which have one valence electron. At the same, it also behaves like halogens as it has just one electron less than the nearest inert gas.
Compare hydrogen with alkali metals on the basis of :
(i) Ion formation
(ii) Reducing power
(iii) Reaction with oxygen
(iv) Oxide formation
Answer
(i) Hydrogen and Alkali metals both form a cation, by loss of an electron.
H ⟶ H+ + e-
Li ⟶ Li+ + e-
Na ⟶ Na+ + e-
All these elements have electropositive character.
(ii) Both Hydrogen atoms and alkali metals are reducing agents.
CuO + H2 ⟶ Cu + H2O
CuO + 2Na ⟶ Cu + Na2O
(iii) Both hydrogen and alkali metals react with oxygen to form respective oxides.
Hydrogen - forms H2O
Sodium - forms Na2O
(iv) Both alkali metals and Hydrogen burns in air to form oxides.
Hydrogen forms its oxide (water)
2H2 + O2 ⟶ 2H2O
Sodium also forms its peroxide
2Na + O2 ⟶ Na2O2
In what respect does hydrogen differ from
(i) Alkali metals
(ii) Halogens
Answer
Properties of hydrogen different from Alkali metals & Halogens are:
- Hydrogen has only one shell but alkali metals and halogens have two or more shells.
- Oxides of hydrogen, H2O, is a neutral oxide. Oxides of halogens like Cl2O, Cl2O7, etc., are acidic in nature, while oxides of alkali metals like Na2O, K2O etc., are basic in nature.
Give the general group study of hydrogen with reference to:
(i) valence electrons
(ii) burning
(iii) reducing power.
Answer
(i) Hydrogen has 1 valence electron in its orbit.
(ii) Hydrogen burns in Oxygen to form oxides (water).
Hydrogen burns with a pop sound.
2H2 + O2 ⟶ 2H2O
(iii) Hydrogen acts as a reducing agent.
CuO + H2 ⟶ Cu + H2O
Why was hydrogen called 'inflammable air'?
Answer
Hydrogen is called inflammable air, because it is a highly combustible gas.
State some sources of hydrogen.
Answer
In free state, hydrogen is found in traces in earth's crust and atmosphere. Volcanic gases contain 0.025% of it, the earth's crust 0.98%, the earth's atmosphere 0.01% and the atmospheres of the Sun and the stars also contain hydrogen in large amounts.
In combined state :
- Plants and animal tissues are made up of compounds of hydrogen with carbon, oxygen and nitrogen.
- Hydrogen is the characteristic constituent of acids, alkalis, hydrocarbons and proteins. In addition to these, sugar, starch, petroleum products, proteins, carbohydrates and also fats contain hydrogen.
- In water, it is 11.1% by weight.
Compare hydrogen and halogens on the basis of :
(i) Physical state
(ii) ion formation
(iii) valency
(iv) reaction with oxygen
Answer
(i) Like halogens (fluorine and chlorine), hydrogen too is a gas.
(ii) Both show a tendency to form anions since they are one electron short of the nearest inert gas configuration.
H + e- ⟶ H-
Cl + e- ⟶ Cl-
(iii) Both have the valency 1.
(iv) Hydrogen reacts with oxygen to form neutral oxides (H2O); Halogen reacts with oxygen to form acidic oxides (Cl2O, Cl2O7 etc.)
Which metal is preferred for the preparation of hydrogen
(i) from water?
(ii) from acid?
Answer
(i) Reactive metals like sodium react with cold water forming their corresponding oxides and evolving hydrogen.
2Na + 2H2O ⟶2NaOH + H2 ↑
Sodium amalgam (alloy of sodium and mercury) react smoothly with water. Therefore, hydrogen can be safely prepared from this amalgam.
(ii) Zinc reacts with acids liberating hydrogen gas and forming their respective salts.
Zn + 2HCl ⟶ ZnCl2 + H2 ↑
(i) Write the reaction of steam with red hot iron.
(ii) Why is this reaction considered a reversible reaction?
(iii) How can the reaction proceed continuously?
Answer
(i) 3Fe + 4H2O ⇌ Fe3O4 + 4H2 ↑
(ii) This reaction is reversible because if the hydrogen formed is not removed, then the iron oxide (triferric tetraoxide) formed is reduced back to iron.
(iii) The hydrogen formed should be removed in order for the reaction to proceed continuously.
Why are zinc and aluminium considered to have a unique nature. Give balanced equations to support your answer.
Answer
Zinc and aluminium are considered to have a unique nature because:
(i) They can react with acids and can even react with hot conc. alkalis to form hydrogen and a soluble salt.
Zn + 2NaOH ⟶ Na2ZnO2 + H2
2Al + 6NaOH ⟶ 2Na3AlO3 + 3H2
Zn + 2HCl ⟶ ZnCl2 + H2
2Al + 6HCl ⟶ 2AlCl3 + 3H2
(ii) Their oxides and hydroxides are amphoteric in nature i.e., they react with both bases and acids to give salt and water.
ZnO + 2HCl ⟶ ZnCl2 + H2O
ZnO + 2NaOH ⟶ Na2ZnO2 + H2O
Al2O3 + 6HCl ⟶ 2AlCl3 + 3H2O
Al2O3 + 2NaOH ⟶ 2NaAlO2 + H2O
Write balanced equations for the following
(i) Iron reacts with dil. HCl
(ii) Zinc reacts with caustic soda solution
(iii) Lead reacts with potassium hydroxide,
(iv) Aluminium reacts with fused sodium hydroxide
Answer
(i) Fe + 2HCl ⟶ FeCl2 + H2
(ii) Zn + 2NaOH ⟶ Na2ZnO2 + H2
(iii) Pb + 2KOH ⟶ K2PbO2 + H2
(iv) 2Al + 6NaOH ⟶ 2Na3AlO3 + 3H2
Write balanced equations and give your observations when the following metals react:
(i) Sodium with cold water
(ii) Calcium with cold water
(iii) Magnesium with boiling water
(iv) Magnesium with steam.
Answer
(i) When Sodium reacts with cold water, it melts, forming a silvery globule, which darts about on the surface of water. It catches fire and burns with a golden yellow flame. Bubbles of hydrogen gas are liberated. The solution formed is colourless, soapy, slightly warm and alkaline.
2Na + 2H2O ⟶2NaOH + H2 ↑
(ii) Calcium sinks in water and the reaction is less vigorous than Sodium. Bubbles of hydrogen gas are liberated and the solution turns milky, turbid and alkaline. If red litmus is introduced in solution, it turns blue.
Ca + 2H2O ⟶ Ca(OH)2 + H2 ↑
(iii) Magnesium reacts slowly with boiling water and forms a base, magnesium hydroxide, liberating hydrogen gas.
Mg + 2H2O ⟶ Mg(OH)2 + H2 ↑
(iv) Magnesium burns in steam with an intense white light, liberating hydrogen gas and white ash which is magnesium oxide.
Mg + H2O ⟶ MgO + H2 ↑
Magnesium oxide crumbles down due to heating. Further exposing magnesium to steam results in the liberation of hydrogen gas.
(i) Under what conditions iron reacts with water?
(ii) Give the balanced equation of the reaction.
(iii) What is noticed if the products are not allowed to escape?
Answer
(i) Red hot iron reacts with steam, forming triferric tetra-oxide and hydrogen gas.
(ii) 3Fe + 4H2O ⇌ Fe3O4 + 4H2 ↑
(iii) If the hydrogen formed is not removed the iron oxide formed is reduced back to iron, as the reaction is reversible.
In the beginning, the forward reaction is fast and the backward reaction is slow. As the products are formed and the reactants get consumed the backward reaction becomes faster. There comes a time when the forward and the backward reactions acquire the same speed. An equilibrium stage is reached at 700°C and the amounts of the reactants and the products do not change.
From the knowledge of activity series, name a metal which shows the following properties:
(i) It reacts readily with cold water
(ii) It displaces hydrogen from hot water
(iii) It displaces hydrogen from dilute HCl
(iv) It forms a base which is insoluble in water.
Answer
(i) Sodium
(ii) Magnesium
(iii) Zinc
(iv) Iron
Complete the following word equations:
(a) Sodium hydroxide + zinc ⟶ hydrogen + ...............
(b) Calcium + water ⟶ calcium hydroxide + ...............
Answer
(a) Sodium hydroxide + zinc ⟶ hydrogen + sodium zincate
(b) Calcium + water ⟶ calcium hydroxide + hydrogen
Hydrogen is evolved by the action of cold dil. HNO3 on
- Fe
- Cu
- Mg
- Zn
Answer
Mg
Reason — Magnesium (Mg) and manganese (Mn) are the only metals that react with very dil. nitric acid to liberate hydrogen. The oxidizing action of the acid is much reduced due to its over dilution.
Mg + 2HNO3 ⟶ Mg(NO3)2 + H2 ↑
Which metal produces hydrogen gas on reaction with cold water?
- Al
- Fe
- Pd
- Na
Answer
Na
Reason — Reactive metals like potassium, sodium and calcium react with cold water forming their corresponding oxides and evolving hydrogen.
2Na + 2H2O ⟶ 2NaOH + H2 ↑
The composition of the nucleus of deuterium is:
- 1e– and 1P
- 1P and 1n
- 1n and 1e
- 2P and 1e–
Answer
1P and 1n
Reason — Hydrogen has three isotopes i.e., namely protium (11H), deuterium (21H) and tritium (31H). Deuterium (21H) has 1 proton and 1 neutron in its nucleus.
Elements which show unique nature in the preparation of hydrogen are
- Na, K, Li
- Mg, Ca, Ba
- Al, Zn, Pb
- Fe, Cu, Ag
Answer
Al, Zn, Pb
Reason — Metals like zinc, aluminium and lead are considered to have a unique nature in the preparation of hydrogen. They react with acids and can even react with hot concentrated alkalis to form hydrogen and a soluble salt.
Hydrogen can be prepared with the metal zinc by using
(i) acid
(ii) alkali
(iii) water
Give an equation in each case.
Answer
(i) Zn + 2HCl ⟶ ZnCl2 (dil.) + H2 ↑
(ii) Zn + 2NaOH ⟶ Na2ZnO2 + H2 ↑
(iii) Zn + H2O ⟶ ZnO + H2 ↑
For laboratory preparation of hydrogen, give the following
(a) materials used
(b) method of collection
(c) chemical equation
(d) fully labelled diagram.
Answer
(a) Granulated zinc, dilute hydrochloric acid or dil. sulphuric acid.
(b) It is collected by the downward displacement of water.
(c) Zn + 2HCl ⟶ ZnCl2 + H2 ↑
(d) Labelled diagram of laboratory preparation of hydrogen:

(a) Name the impurities present in hydrogen prepared in the laboratory.
(b) How can these impurities be removed?
Answer
(a) Impurities present are:
- Hydrogen sulphide (H2S)
- Sulphur dioxide (SO2)
- Oxides of nitrogen
- Phosphine (PH3)
- Arsine (AsH3)
- Carbon dioxide and
- Water vapour
(b) The impurities can be removed from hydrogen by passing it through:
- Silver nitrate solution [to remove arsine and phosphine].
AsH3 + 6AgNO3 ⟶ Ag3As + 3AgNO3 + 3HNO3
PH3 + 6AgNO3 ⟶ Ag3P + 3AgNO3 + 3HNO3 - Lead nitrate solution [to remove hydrogen sulphide].
Pb(NO3)2 + H2S ⟶ PbS + 2HNO3 - Caustic potash solution [to remove sulphur dioxide, carbon dioxide and oxides of nitrogen].
SO2 + 2KOH ⟶ K2SO3 + H2O
CO2 + 2KOH ⟶ K2CO3 + H2O
2NO2 + 2KOH ⟶ KNO2 + KNO3 + H2O - A drying agent used to dry the gas. Common drying agents such as fused calcium chloride, caustic potash stick and phosphorus pentoxide remove water vapour.
Thus, the gas is purified and dried and then collected over mercury because mercury has no reaction with it.
Which test should be made before collecting hydrogen in a gas jar?
Answer
The gas should be collected only after all the air in the apparatus has escaped. This can be ascertained by collecting some amount of gas in a test tube and taking it to a flame. If the gas burns quietly, then there is no more air in the flask.
Why is nitric acid not used in the preparation of hydrogen?
Answer
As nitric acid is a powerful oxidizing agent, and the oxygen formed due to its decomposition oxidizes the hydrogen to give water thus defeating the purpose of the reaction. Hence, Nitric acid is not used in the preparation of hydrogen.
3Zn + 8HNO3 ⟶ 3Zn(NO3)2 + 4H2O + 2NO ↑
Why is hot concentrated sulphuric acid not used in the preparation of hydrogen?
Answer
Hot concentrated sulphuric acid is not used in the preparation of hydrogen as it is a strong oxidizer and will produce sulphur dioxide.
Zn + 2H2SO4 ⟶ ZnSO4 + SO2 + 2H2O
Hydrogen is manufactured by 'Bosch Process'.
(a) Give the equations with conditions.
(b) How can you obtain hydrogen from a mixture of Hydrogen and carbon monoxide?
Answer
(a) Steam is passed over hot coke (1000°C) in furnaces of a special design, called converters, giving water gas.
The reaction is endothermic.
(b) Water gas i.e., hydrogen and carbon monoxide is mixed with excess steam and passed over heated ferric oxide, which acts as a catalyst and chromic oxide Cr2O3 which acts as a promoter.
Give equations to express the reaction between:
(a) steam and red hot iron
(b) calcium and water
Answer
(a) 3Fe + 4H2O ⇌ Fe3O4 + 4H2 ↑
(b) Ca + 2H2O ⟶ Ca(OH)2 + H2 ↑
A small piece of calcium metal is put into a small trough containing water. There is effervescence and white turbidity is formed.
(a) Name the gas formed in the reaction. How would you test the gas?
(b) Write an equation for the reaction.
(c) What do you observe when a few drops of red litmus solution are added to the turbid solution?
Answer
(a) Hydrogen gas.
It burns silently in air or oxygen with a pale blue flame, forming water.
2H2 + O2 ⟶ 2H2O
When it is premixed with air or oxygen, it explodes with a pop sound.
(b) Ca + 2H2O ⟶ Ca(OH)2 + H2 ↑
(c) The solution turns blue.
Thin strips of magnesium, copper and iron are taken.
(a) Write down what happens when these metals are treated as follows
(i) Heated in presence of air
(ii) Heated with dil. HCl
(iii) Added to an aqueous solution of zinc sulphate
(b) Arrange these metals in descending order of reactivity.
Answer
(a) (i) When we heat magnesium, copper and iron in presence of air they form respective oxides.
(ii) Magnesium and iron react with HCl liberating hydrogen and forming their respective salts.
Mg + 2HCl ⟶ MgCl2 + H2 ↑
Fe + 2HCl ⟶ FeCl2 + H2 ↑
As Cu is below hydrogen in metal reactivity series, hence, it cannot displace hydrogen from acid and so no reaction takes place.
Cu + HCl (dil) ⟶ no reaction
(iii) Magnesium will displace zinc from zinc sulphate solution as magnesium is more reactive than zinc and is placed above zinc in the reactivity series of metals.
Mg + ZnSO4 ⟶ MgSO4 + Zn
Copper and iron are less reactive and are placed below zinc in the reactivity series, hence will not displace it and no reaction will take place.
(b) Mg > Fe > Cu
Give the reason for the following
(a) Zinc granules are used in the lab preparation of hydrogen.
(b) Purified and dried hydrogen is collected over mercury.
(c) The end of the thistle funnel should be dipped under acid.
(d) Dilute sulphuric acid cannot be replaced by concentrated acid in the preparation of hydrogen.
Answer
(a) Granulated zinc contains traces of impurities like copper, which has a slight catalyzing effect on the reaction and speeds it up. Hence, granulated zinc is preferred in the lab preparation of hydrogen over pure zinc.
(b) Hydrogen gas is purified and dried and then collected over mercury because mercury has no reaction with it.
(c) The end of the thistle funnel should be dipped under acid so as to prevent gas from escaping the thistle funnel.
(d) Dilute Sulphuric acid cannot be replaced by concentrated acid in the preparation of hydrogen as it is a strong oxidiser and it will produce sulphur dioxide.
Zn + 2H2SO4 ⟶ ZnSO4 + SO2 + 2H2O
(a) Where does Hydrogen occur in free state?
(b) How did the name 'hydrogen' originate?
Answer
(a) In free state, hydrogen is found in traces in earth's crust and atmosphere. Volcanic gases contain 0.025% of it, the earth's crust 0.98%, the earth's atmosphere 0.01% and the atmospheres of the Sun and the stars also contain hydrogen in large amounts.
(b) It is on account of its ability to form water that Lavoisier, in 1783, named it hydrogen (Greek word meaning water former).
Hydrogen can be prepared with the help of cold water. Give a reaction of hydrogen with:
(a) a monovalent metal
(b) divalent metal
Answer
(a) 2K + H2 2KH
(b) Ca + H2 CaH2
Which metal is preferred for preparing hydrogen from:
(a) cold water?
(b) hot water?
(c) steam?
Write balanced equation for each case.
Answer
(a) Calcium is preferred for preparing hydrogen from cold water as the reaction is less vigorous than Sodium.
Ca + 2H2O ⟶ Ca(OH)2 + H2 ↑
(b) Magnesium reacts with boiling water and forms a base, magnesium hydroxide, liberating hydrogen gas.
Mg + 2H2O ⟶ Mg(OH)2 + H2 ↑
(c) Zinc is preferred for preparing hydrogen from steam. When steam is passed over heated zinc, hydrogen is liberated and zinc is converted to zinc oxide.
Zn + H2O ⟶ ZnO + H2 ↑
Hydrogen may be prepared in the laboratory by the action of a metal with an acid.
(a) Which of the metals copper, zinc, magnesium or sodium would be the most suitable?
(b) Which of the acids dilute sulphuric, concentrated sulphuric, dilute nitric and concentrated nitric would you choose? Explain why you would not use the acids you rejected.
(c) How would you modify your apparatus to collect dry hydrogen? Which drying agent would you employ for this purpose?
Answer
(a) Zinc, because sodium reacts violently with acid, magnesium is expensive and copper does not displace hydrogen when reacted with acids as it lies below hydrogen in the metal activity series.
(b) Dilute sulphuric acid is preferred to prepare hydrogen in the laboratory because:
- Nitric acid, even in its dilute form, is not used in the preparation of hydrogen from metals because it is a powerful oxidizing agent, and the oxygen formed due to its decomposition oxidizes the hydrogen to give water, thus defeating the purpose of the reaction.
3Zn + 8HNO3 ⟶ 3Zn(NO3)2 + 4H2O + 2NO↑ - The reason for not using concentrated sulphuric acid is that it is a strong oxidiser and will produce sulphur dioxide.
Zn + 2H2SO4 ⟶ ZnSO4 + SO2 + 2H2O
(c) The purified and dry hydrogen gas is collected over mercury because mercury has no reaction with it.
Common drying agents like fused calcium chloride, caustic potash stick and phosphorous pentoxide can be used to dry hydrogen gas.
Why are the following metals not used in the lab. preparation of hydrogen?
(a) calcium
(b) iron
(c) aluminium
(d) sodium
Answer
The following metals are not used in the lab. preparation of hydrogen because:
(a) Calcium is expensive.
(b) Iron has to be heated, but then the hydrogen thus produced contains impurities like hydrogen sulphide and sulphur dioxide.
(c) Aluminium forms a protective coating of Al2O3 due to its great affinity for oxygen. Due to the coating of Al2O3, aluminium does not give hydrogen with acid.
(d) Sodium reacts violently with acid.
Based on the reactions of water on metals, arrange the following metals in increasing order of reactivity: Iron, sodium, magnesium, zinc, calcium.
Answer
Iron < Zinc < Magnesium < Calcium < Sodium
Hydrogen is evolved when dilute HCl reacts with magnesium, but nothing happens in the case of mercury and silver. Explain.
Answer
As magnesium is more reactive than hydrogen and is placed above it in the metal reactivity series, hence hydrogen is displaced by magnesium but mercury and silver are less reactive than hydrogen and are placed below it in the series, hence they can't displace hydrogen.
Steam can react with a metal and a non-metal to liberate hydrogen. Give the necessary conditions and equations for the same.
Answer
The conditions necessary for the reaction of steam with a metal and a non-metal to liberate hydrogen are:
- Presence of steam — Steam (water vapor) is required for this reaction to occur.
- Heat — The reaction is typically carried out at high temperatures to facilitate the reaction between the metal or non-metal and steam.
Reaction of Zinc (metal) with steam to liberate hydrogen:
Reaction of Carbon (non-metal) with steam to liberate hydrogen:
Hydrogen is obtained by displacement from
(a) dilute sulphuric acid
(b) dilute hydrochloric acid
Write equations using zinc and iron.
Why does not copper show similar behaviour?
Answer
(a) dilute sulphuric acid
Zn + H2SO4 (dil.) ⟶ ZnSO4 + H2 ↑
Fe + H2SO4 (dil.) ⟶ FeSO4 + H2 ↑
(b) dilute hydrochloric acid
Zn + 2HCl (dil.) ⟶ ZnCl2 + H2 ↑
Fe + 2HCl (dil.) ⟶ FeCl2 + H2 ↑
Zinc and iron are above hydrogen in activity series of metals whereas copper is below hydrogen in the series. As metals below hydrogen cannot displace it from dilute acids hence, copper does not show similar behaviour as zinc and iron.
Give a reason for the following
(a) Though lead is above hydrogen in the activity series, it is not used to prepare hydrogen.
(b) Potassium and sodium are not used for reaction with dilute hydrochloric acid or dilute sulphuric acid in the laboratory preparation of hydrogen.
Answer
(a) Lead reacts with dilute sulphuric acid or hydrochloric acid and forms an insoluble coating of lead sulphate or lead chloride. Therefore, further reaction is prevented. Hence, it is not used to prepare hydrogen.
(b) Potassium and sodium react violently with acid, hence they are not used for reaction with dilute hydrochloric acid or dilute sulphuric acid in the laboratory preparation of hydrogen.
Name two alkalies that can displace hydrogen. Give balanced equations for the same. Why are the metals you have used considered to have unique nature?
Answer
NaOH and KOH
Zn + 2NaOH ⟶ Na2ZnO2 + H2 ↑
Zn + 2KOH ⟶ K2ZnO2 + H2 ↑
Zinc and aluminium are considered to have a unique nature because:
(i) They can react with acids and can even react with hot conc. alkalis to form hydrogen and a soluble salt.
Zn + 2HCl ⟶ ZnCl2 + H2
(ii) Their oxides and hydroxides are amphoteric in nature i.e., they react with both bases and acids to give salt and water.
ZnO + 2HCl ⟶ ZnCl2 + H2O
ZnO + 2NaOH ⟶ Na2ZnO2 + H2O
Complete and balance the following equations.
(a) Na + H2O ⟶ ............... + ...............
(b) Ca + H2O ⟶ ............... + ...............
(c) Mg + H2O ⟶ ............... + ...............
(d) Zn + H2O ⟶ ............... + ...............
(e) Fe + H2O ⟶ ............... + ...............
(f) Zn + HCl ⟶ ............... + ...............
(g) Al + H2SO4 ⟶ ............... + ...............
(h) Fe + HCl ⟶ ............... + ...............
(i) Zn + NaOH ⟶ ............... + ...............
(j) Al + KOH + H2O ⟶ ............... + ...............
Answer
(a) 2Na + 2H2O ⟶ 2NaOH + H2
(b) Ca + 2H2O ⟶ Ca(OH)2 + H2
(c) Mg + 2H2O ⟶ Mg(OH)2 + H2
(d) Zn + H2O ⟶ ZnO + H2
(e) 3Fe + 4H2O ⇌ Fe3O4 + 4H2
(f) Zn + 2HCl ⟶ ZnCl2 + H2
(g) 2Al + 3H2SO4 ⟶ Al2(SO4)3 + 3H2
(h) Fe + 2HCl ⟶ FeCl2 + H2
(i) Zn + 2NaOH ⟶ Na2ZnO2 + H2
(j) 2Al + 2KOH + 2H2O ⟶ 2KAlO2 + 3H2
If the following are kept in closed vessels at over 400°C what would happen to them ?
(a) iron filings and steam,
(b) hydrogen and magnetic oxide of iron ?
Answer
(a) Iron oxide is formed with liberation of hydrogen gas.
3Fe + 4H2O ⇌ Fe3O4 + 4H2 ↑
(b) Hydrogen when passed over heated magnetic oxide of iron, it reduces the oxide to free metal.
Fe2O3 + 3H2 ⟶ 2Fe + 3H2O
(a) A metal in the powdered form reacts very slowly with the boiling water, but it decomposes with steam. Name the metal.
(b) Write a balanced equation for the reaction of the named metal with (i) boiling water (ii) steam.
Answer
(a) Magnesium
(b) The reaction of Magnesium with:
- boiling water
Mg + 2H2O ⟶ Mg(OH)2 + H2 ↑ - steam
Mg + H2O [steam] ⟶ MgO + H2 ↑
What do you observe when hydrogen gas is passed through soap solution?
Answer
On passing hydrogen gas through the soap solution, soap bubbles filled with hydrogen start coming out of the solution and rise up in the air. This behaviour proves that hydrogen is lighter than air.
Under what conditions can hydrogen be made to combine with
(a) nitrogen
(b) chlorine
(c) sulphur
(d) oxygen
Name the products in each case and write the equation for each reaction.
Answer
(a) Three volumes of hydrogen and one volume of nitrogen react at temperature 450-500°C, pressure 200-900 atm in the presence of a catalyst, finely divided iron, with molybdenum as a promoter to give ammonia.
(b) Equal volumes of hydrogen and chlorine react slowly in diffused sunlight but explosively in direct sunlight, to form hydrogen chloride.
(c) Hydrogen gas on passing through molten sulphur reacts to give hydrogen sulphide.
(d) Hydrogen burns (in presence of electric spark) with a 'pop' sound in oxygen and with a blue flame forming water.
When hydrogen is passed over a black solid compound A, the products are 'a colourless liquid' and 'a reddish-brown metal B.'
Substance B is divided into two parts, each placed in separate test tubes.
Dilute HCl is added to one part of substance B and dilute HNO3 to the other.
(a) Name the substances A and B.
(b) Give two tests for the colourless liquid formed in the experiment.
(c) What happens to substance A when it reacts with hydrogen ? Give reasons for your answer.
(d) Write an equation for the reaction between hydrogen and substance A.
(e) Is there any reaction between substance B and dilute hydrochloric acid ? Give reasons for your answer.
Answer
(a) A = copper oxide [CuO],
B = copper [Cu]
(b) The liquid does not change the colour of blue or red litmus paper, showing neutral nature.
It changes the colour of cobalt chloride paper from blue to pink colour. Hence, the liquid produced is water.
(c) Black copper oxide (A) is reduced to copper, by losing oxygen. This happens because, hydrogen, the reducing agent reduces copper oxide to copper.
(d) CuO + H2 Cu + H2O
(e) As copper is less reactive than hydrogen and lies below it in the metal reactivity series, hence it cannot displace it from HCl. Hence, there is no reaction between them.
Cu + HCl ⟶ No reaction
Helium is preferred to hydrogen for filling balloons because it is
- lighter than air
- almost as light as hydrogen
- non-combustible
- inflammable
Answer
non-combustible
Reason — Hydrogen is also lighter than air but it is a combustible gas. It forms an explosive mixture with air (due to the oxygen present in it) and to avoid this risk helium gas is preferred.
Upon reacting with water, an active metal produces
- oxygen
- nitric acid
- a base
- none of these.
Answer
a base
Reason — Active metals like potassium, sodium and calcium react with cold water forming their corresponding hydroxides (i.e., bases) and evolving hydrogen.
A metal oxide that is reduced by hydrogen is:
- Al2O3
- CuO
- CaO
- Na2O
Answer
CuO
Reason — Copper oxide loses oxygen, thus reduction of CuO to Cu take place.
CuO + H2 ⟶ Cu + H2O
Which of the following statements about hydrogen is incorrect ?
- It is an inflammable gas
- It is the lightest gas.
- It is not easily liquefied.
- It is a strong oxidizing agent.
Answer
It is a strong oxidizing agent.
Reason — Hydrogen is not a strong oxidizing agent. It is a reducing agent. It reduces the oxides of the less active metals i.e., it removes oxygen from strongly heated metal oxides when passed over them and itself gets oxidized to water.
For the reaction PbO + H2 ⟶ Pb + H2O, which of the following statements is wrong?
- H2 is the reducing agent.
- PbO is the oxidizing agent.
- PbO is oxidized to Pb
- H2 is oxidized to H2O.
Answer
PbO is oxidized to Pb
Reason — PbO is reduced to Pb, as oxygen has been removed from it.
Which metal gives hydrogen with all of the following: water, acids, alkalis?
- Fe
- Zn
- Mg
- Pb
Answer
Zn
Reason — Zn has a unique nature.
Zinc is considered to have a unique nature because it can react with acids and can even react with hot conc. alkalis to form hydrogen and a soluble salt.
Zn + 2HCl ⟶ ZnCl2 + H2
Zn + 2NaOH ⟶ Na2ZnO2 + H2 ↑
Zn with water also gives hydrogen
Zn + H2O ⟶ ZnO + H2
Which of the following metals does not give hydrogen with acids?
- Iron
- Copper
- Magnesium
- Zinc
Answer
Copper
Reason — Hydrogen cannot be prepared from metals that are below in the metal reactivity series such as copper, since only metals that are more reactive than hydrogen can displace it from acids.
Which of the following statements about the reaction given below are correct?
3Fe (s) + 4H2O (g) ⟶ Fe3O4 (s) + 4H2 (g)
P — Iron is getting oxidised.
Q — Water is getting reduced.
R — Water is a reducing agent.
S — Water is an oxidising agent.
- P, Q and R
- R and S
- P, Q and S
- Q and S
Answer
P, Q and S
Reason — In the above reaction, steam is passed over red hot iron. Oxygen has been added to iron, iron has been oxidised by water vapour. Therefore, water is oxidising agent and water is getting reduced by iron, which is reducing agent.
Which of the following statements about the reaction given below are incorrect?
ZnO (s) + C (s) ⟶ Zn (s) + CO
P — Zinc is getting reduced.
Q — Carbon is getting oxidised.
R — Carbon monoxide is getting oxidised.
S — Zinc oxide is getting reduced.
- P and Q
- P and R
- P, Q and R
- All are incorrect
Answer
P and R
Reason — The above reaction is an example of reaction between oxide and element, which gives metal. Here zinc (II) oxide is reduced to zinc by losing oxygen. Whereas carbon is getting oxidised by the gain of oxygen. Hence P and Q are incorrect.
Zinc metal can react with which of the following options under appropriate conditions to produce hydrogen?
P — Steam
Q — Dilute acid
R — Strong alkalies
- Only P
- Q and R
- R and P
- All of these
Answer
All of these
Reason
(i) Zinc reacts with steam.
Zn + H2O ⟶ ZnO + H2
(ii) Zinc reacts with dilute acid.
Zn + 2HCl ⟶ ZnCl2 + H2
(iii) Zinc reacts with strong alkali.
Zn + 2NaOH ⟶ Na2ZnO2 + H2
The table given below shows the reaction of a few elements with acids and bases to evolve hydrogen gas.
| Element | Acid | Base |
|---|---|---|
| (A) | X | X |
| (B) | ✓ | ✓ |
| (C) | ✓ | X |
| (D) | ✓ | ✓ |
Which of these elements will form amphoteric oxides.
- A and D
- B and D
- A and C
- B and D
Answer
B and D
Reason — In the above table, elements (B) and (D), when reacted with both acid and base, evolve hydrogen gas. So, they will form amphoteric oxides.
Assertion (A): Though hydrogen is lighter than air, it is not collected by the downward displacement of air but by the downward displacement of water.
Reason (R): Hydrogen is slightly soluble in water.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Hydrogen is collected by the downward displacement of water because it forms an explosive mixture with air and therefore cannot be collected by downward displacement of air even though it is lighter than air. Hence the assertion (A) is true.
Hydrogen is virtually insoluble in water (does not dissolve in water). Hence the reason (R) is false.
Assertion (A): Hydrogen and alkali metals react with a black copper oxide to give red/brown copper.
Reason (R): Hydrogen and alkali metals are good oxidising agents.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Hydrogen and alkali metals react with copper oxide to give red/ brown copper.
CuO + H2 ⟶ Cu + H2O
CuO + 2Na ⟶ Cu + Na2O
Hence assertion (A) is true.
Both hydrogen and alkali metals acts as reducing agents. Hence reason (R) is False.
Assertion (A): Granulated zinc is preferred over pure zinc in the preparation of hydrogen using dilute acids.
Reason (R): Granulated zinc contains traces of impurities which have a slight catalysing effect on the reaction.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Zinc granules are preferred for preparation of hydrogen over pure zinc because the impurity present in granulated zinc is copper, whose catalysing effect speeds up the rate of reaction. Hence both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): In Bosch process, hydrogen and carbon dioxide are formed.
Reason (R): Hydrogen and carbon dioxide are separated by passing through ammoniacal cuprous chloride solution.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — In the second step of Bosch process, water gas is mixed with excess steam and passed over heated ferric oxide, which acts as a catalyst and chromic oxide Cr2O3, which acts as a promoter to produce hydrogen and carbon dioxide.
Hence assertion (A) is true.
The mixture Hydrogen and carbon dioxide(CO2 + H2) are separated through caustic potash solution, which removes carbon dioxide by reacting with it, leaving hydrogen. Hence reason (R) is false.
Assertion (A): While preparing hydrogen in laboratory, no leakage of gas should take place and no flame must be present near the apparatus.
Reason (R): Hydrogen-air mixture burns with a characteristic pop sound.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true but R is not the correct explanation of A.
Explanation — While preparing hydrogen in laboratory, no leakage of gas should take place and no flame must be present near the apparatus because the mixture of hydrogen and air explodes violently when it is brought near a flame. Hence assertion (A) is true.
When hydrogen is pre-mixed with air or oxygen, it explodes with a pop sound. Hence reason (R) is true.
But reason (R) does not explain why no leakage of gas and no flame must be maintained in laboratory. Hence reason (R) is not correct explanation of assertion (A).
Assertion (A): Hydrogen shows a dual nature.
Reason (R): Hydrogen resembles both the alkali metals of group 1A and the halogens of group VIIA.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Hydrogen shows a dual nature. Hydrogen resembles the alkali metals of Group IA and the halogens of Group VIIA. This is due to the fact that it has only one electron in its valence shell, hence it behaves like the alkali metals which have one valence electron. At the same, it also behaves like halogens as it has just one electron less than the nearest inert gas. Hence both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): Both dilute sulphuric acid and dilute hydrochloric acid displace hydrogen when they react with metals above hydrogen in the activity series.
Reason (R): The more reactive element displaces the less reactive element from its salt solution.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Metals which are more reactive than hydrogen, when reacted with both dilute sulphuric acid and dilute hydrochloric acid displace hydrogen. The extent to which these reaction occur for a given metal is also based on the activity series of metal where the more reactive element displaces the less reactive element from its salt solution. Hence both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): Zinc, lead and aluminium are unique metals.
Reason (R): Zinc, lead and aluminium all have the same valency.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Metals like zinc, lead and aluminium have a unique nature because they react with acids and can even react with hot concentrated alkalis to form hydrogen and a soluble salt. Hence, the assertion (A) is true.
Zinc, lead and aluminium have different valencies. Zinc and lead have +2 valency and aluminium has +3 valency. Hence, the reason (R) is false.
Assertion (A): Zinc is preferred for the laboratory preparation of hydrogen.
Reason (R): Zinc is unique in nature and does not contain any impurity.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Zinc granules are preferred for laboratory preparation of hydrogen over pure zinc because the impurity present in granulated zinc is copper, whose catalysing effect speeds up the rate of reaction. Hence the assertion (A) is true.
Zinc is unique in nature and does contain impurity like copper. Hence reason (R) is false.
Assertion (A): Reduction is defined as the process of removal of oxygen from a substance or the addition of hydrogen to a substance.
Reason (R): Hydrogen is a reducing as well as an oxidising agent.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Reduction is also defined as the process that involves removal of oxygen from a substance or the addition of hydrogen to a substance. Hence, the assertion (A) is true.
Hydrogen is a reducing agent not oxidising agent. Hence, the reason (R) is false.
Choose terms from the options given in brackets to complete these sentences.
(a) When CuO reacts with hydrogen, ............... is reduced to ............... and ............... is oxidized to ............... . (CuO, H2, Cu, H2O)
(b) Hydrogen is ............... soluble in water. (sparingly, highly, moderately)
(c) Metals like ..............., ............... and ............... give H2 with steam. (iron, magnesium, aluminium, sodium, calcium)
(d) Sodium ............... reacts smoothly with cold water. (metal, amalgam, in the molten state)
(e) A metal ............... hydrogen in the activity series gives hydrogen with ............... acid or ............... acid. (above, below, dilute hydrochloric, concentrated hydrochloric, dilute sulphuric).
Answer
(a) When CuO reacts with hydrogen, CuO is reduced to Cu and H2 is oxidized to H2O
(b) Hydrogen is sparingly soluble in water.
(c) Metals like magnesium, iron and aluminium give H2 with steam.
(d) Sodium amalgam reacts smoothly with cold water.
(e) A metal above hydrogen in the activity series gives hydrogen with dilute hydrochloric acid or dilute sulphuric acid acid.
Name
(a) an oxidizing agent that does not contain oxygen.
(b) a substance that oxidizes concentrated HCl to chlorine.
(c) a substance that will reduce aqueous Iron (III) ions to Iron (II) ions.
(d) a liquid that is an oxidizing as well as a reducing agent.
(e) a gas that is an oxidizing as well as a reducing agent.
(f) a solid that is an oxidizing agent.
Answer
a) Chlorine [Cl2]
b) Manganese dioxide [MnO2]
c) Hydrogen sulphide [H2S]
(d) Hydrogen peroxide [H2O2]
(e) Hydrogen [H2]
(f) Potassium permanganate [KMnO4]
Correct the following statements:
(a) Hydrogen is separated from CO by passing the mixture through caustic potash solution.
(b) All metals react with acids to give hydrogen.
(c) Hydrogen is dried by passing it through conc. H2SO4.
(d) Very dilute nitric acid reacts with iron to produce hydrogen.
(e) Conc. H2SO4 reacts with zinc to liberate hydrogen.
Answer
(a) Hydrogen is separated from CO2 by passing the mixture through caustic potash solution.
(b) All the metals above hydrogen in the metal activity series react with acids to give hydrogen.
(c) Hydrogen is dried by passing it through fused calcium chloride.
(d) Very dilute nitric acid reacts with magnesium [Mg] and manganese [Mn] to produce hydrogen.
(e) Dilute H2SO4 reacts with zinc to liberate hydrogen.
Divide the following redox reactions into oxidation and reduction half reactions.
(i) Zn + Pb2+ ⟶ Zn2+ + Pb
(ii) Zn + Cu2+ ⟶ Zn2+ + Cu
(iii) Cl2 + 2Br- ⟶ Br2 + 2Cl-
Answer
(i) Zn + Pb2+ ⟶ Zn2+ + Pb
Oxidation : Zn ⟶ Zn2+ + 2e-
Reduction : Pb2+ + 2e- ⟶ Pb
(ii) Zn + Cu2+ ⟶ Zn2+ + Cu
Oxidation : Zn ⟶ Zn2+ + 2e-
Reduction : Cu2+ + 2e- ⟶ Cu
(iii) Cl2 + 2Br- ⟶ Br2 + 2Cl-
Oxidation : Cl2 ⟶ 2Cl- + 2e-
Reduction : 2Br- + 2e- ⟶ Br2
(a) Write the equation in the ionic form
CuSO4 (aq) + Fe (s) ⟶ FeSO4 (aq) + Cu (s)
(b) Divide the above equation into oxidation and reduction half reactions.
Answer
(a) The equation in the ionic form:
Cu2+ SO42- + Fe ⟶ Fe2+ SO42- + Cu
(b) Oxidation : Fe ⟶ Fe2+ + 2e-
Reduction : Cu2+ + 2e- ⟶ Cu
Select the odd one out and justify your answer.
(a) Zn, Fe, Mg and Na
(b) SO2, H2S, NH3 and CO2
(c) Fe, Zn, Cu and Mg
(d) Fe, Pb, Al and Zn
Answer
(a) Na, as it reacts with cold water to produce hydrogen gas while others react with hot water/steam to produce hydrogen gas.
(b) NH3, as it is basic in nature but rest are acidic.
(c) Cu, as it is below hydrogen in the metal activity series, hence it cannot displace hydrogen from dilute acids whereas others are above hydrogen and can displace it from dilute acids.
(d) Fe as rest of the elements Pb, Al and Zn have unique nature. They can react with acids as well as hot concentrated alkalis to form hydrogen and a soluble salt.
Give reasons
(a) Hydrogen is collected by the downward displacement of water and not of air, even though it is lighter than air.
(b) A candle brought near the mouth of a jar containing hydrogen gas starts burning but is extinguished when pushed inside the jar.
(c) Apparatus for laboratory preparation of hydrogen should be air tight and away from a naked flame.
Answer
(a) Hydrogen is collected by the downward displacement of water as it forms an explosive mixture with air and therefore, cannot be collected by downward displacement of air even though it is lighter than air.
(b) Hydrogen is a combustible gas but it does not support the combustion process, hence, when a candle is brought near the mouth of a jar containing hydrogen gas, it starts burning but is extinguished when pushed inside the jar.
(c) Apparatus for laboratory preparation of hydrogen should be air tight becasue hydrogen forms an explosive mixture with air. Hydrogen is a highly flammable gas, if a naked flame is present near the apparatus, it can lead to an ignition of the hydrogen, resulting in an explosion.
Write half-reactions for the following reaction :
A+ + B ⟶ A + B+ and name the following:
(a) oxidizing agent
(b) substance oxidized
(c) reducing agent.
Answer
Half reaction:
A+ + e- ⟶ A (Reduction)
B ⟶ B + e- (Oxidation)
a. A, as it is oxidizing B by accepting electrons.
b. B, as it is losing electrons.
c. B, a it is reducing A by providing electrons.
State whether the following conversions are oxidation or reduction reactions.
(a) PbO2 + SO2 ⟶ PbSO4
(b) Cu2+ + 2e- ⟶ Cu
(c) K ⟶ K+ + e-
(d) 2Cl- - 2e- ⟶ Cl2
Answer
(a) Oxidation, gain of oxygen
(b) Reduction, gain of electron
(c) Oxidation; loss of electron
(d) Oxidation, loss of electron
State, giving reasons, whether the substances printed in bold letters have been oxidised or reduced.
(a) PbO + CO ⟶ Pb + CO2
(b) Mg + 2HCl ⟶ MgCl2 + H2
(c) H2S + Cl2 ⟶ 2HCl + S
(d) Cl2 + H2S ⟶ 2HCl + S
Answer
(a) PbO is reduced, due to removal of oxygen.
(b) Mg is oxidised, due to loss of electrons.
(c) H2S is oxidised, as hydrogen is lost to give sulphur.
(d) Chlorine is reduced, due to addition of hydrogen to form HCl.
Is it essential that oxidation and reduction must occur side by side in a chemical reaction? Explain.
Answer
In a chemical reaction, if one substance is oxidized, the other substance must necessarily be reduced. This is because the electrons lost during oxidation are simultaneously gained during reduction and vice versa.
For example, Zinc reacts with copper sulphate to form zinc sulphate and copper.
CuSO4 + Zn ⟶ ZnSO4 + Cu
Cu2+ SO42- + Zn ⟶ Zn2+ SO42- + Cu
Writing half-reaction,
Zn ⟶ Zn2+ + 2e- (Oxidation)
Cu2+ + 2e- ⟶ Cu (Reduction)
They occur simultaneously as
Cu2+ + Zn ⟶ Zn2+ + Cu
Thus, oxidation and reduction always occur simultaneously in a chemical reaction.
Describe briefly the ionic concept of oxidation and reduction. Give an equation to illustrate.
Answer
Oxidation is a process in which an atom or ion loses an electron(s).
Example: Zn ⟶ Zn2+ + 2e-
Reduction is a process in which an atom or ion gains electron(s).
Example: Cu2+ + 2e- ⟶ Cu (Reduction)
State the similarities of hydrogen with group 1 elements and group 17 elements.
Answer
Similarity of hydrogen with group 1 elements:
- Electronic configuration — Both hydrogen and alkali metals have only one electron in their outermost orbits.
- Ion formation — Both form a cation by loss of an electron.
H ⟶ H+ + e-
Na ⟶ Na+ + e- - Valency — Both have the valency 1.
- Reducing power — Both act as reducing agents.
CuO + H2 ⟶ Cu + H2O
CuO + 2Na ⟶ Cu + Na2O - Reaction with Oxygen — Both react with oxygen to form respective oxides.
Hydrogen forms H2O
Sodium forms Na2O
Similarity of hydrogen with group 17 elements:
- Electronic configuration — Hydrogen and halogens have one electron less than the nearest inert gas.
- Physical state — Like halogens (fluorine and chlorine), hydrogen too is a gas.
- Ion formation — Both form an anion by accepting an electron.
H + e- ⟶ H-
Cl + e- ⟶ Cl- - Valency — Both have the valency 1.
- Atomicity — Hydrogen as well as halogens exist in the form of diatomic molecules (H2, F2, Cl2, Br2, I2).
Which is the most preferred metal for the laboratory preparation of hydrogen. Why is any other metal not used?
Answer
Zinc is the most preferred metal in the laboratory preparation of hydrogen.
Other metals are not used because of the following reasons:
- Sodium and potassium react violently with acid.
- Calcium and magnesium are expensive.
- Aluminium forms a protective coating of Al2O3 due to its great affinity for oxygen. Due to the coating of Al2O3, aluminium does not give hydrogen with acid.
- Iron has to be heated, but then the hydrogen thus produced contains impurities like hydrogen sulphide and sulphur dioxide.
- Lead reacts with dil. sulphuric acid or hydrochloric acid and forms an insoluble coating of lead sulphate or lead chloride. Therefore, further reaction is prevented.
- Hydrogen cannot be prepared from metals that are below in the metal reactivity series such as copper and mercury, since only metals that are more reactive than hydrogen can displace it from acids.
Describe the laboratory preparation of hydrogen with a labelled diagram? How are the impurities removed.
Answer
Reactants : Granulated zinc, dil. hydrochloric acid or dil. sulphuric acid.
Procedure : Place some pieces of granulated zinc in a flat bottom flask fitted with an air tight cork with two holes. Through one hole, pass a thistle funnel with a long stem provided with a stopper, and through the other, a long delivery tube.
Pour dilute hydrochloric acid (or dilute sulphuric acid) through the funnel.

Zn + 2HCl (dil.) ⟶ ZnCl2 + H2 ↑
Zn + H2SO4 (dil.) ⟶ ZnSO4 + H2 ↑
Observation : Reaction will gradually start in the form of effervescence and evolution of gas. When all the air from the apparatus has been expelled, collect the gas over water by downward displacement of water.
Impurities can be removed from hydrogen by passing it through :
- Silver nitrate solution [to remove arsine and phosphine].
AsH3 + 6AgNO3 ⟶ Ag3As + 3AgNO3 + 3HNO3
PH3 + 6AgNO3 ⟶ Ag3P + 3AgNO3 + 3HNO3 - Lead nitrate solution [to remove hydrogen sulphide].
Pb(NO3)2 + H2S ⟶ PbS + 2HNO3 - Caustic potash solution [to remove sulphur dioxide, carbon dioxide and oxides of nitrogen].
SO2 + 2KOH ⟶ K2SO3 + H2O
CO2 + 2KOH ⟶ K2CO3 + H2O
2NO2 + 2KOH ⟶ KNO2 + KNO3 + H2O - A drying agent used to dry the gas. Common drying agents such as fused calcium chloride, caustic potash stick and phosphorus pentoxide remove water vapour.
Thus, the gas is purified and dried and then collected over mercury because mercury has no reaction with it.
How is hydrogen manufactured? Describe with the equation(s) involved.
Answer
Hydrogen is manufactured by Bosch process. It consists of following steps:
1. Production of water gas
Reactants : White hot coke and steam
Temperature : Around 1000°C
Process : Passage of steam over white hot coke [carbon]
Chamber : Specially designed convertor
2. Reduction of steam to hydrogen by carbon monoxide
Reactants : Water gas and excess steam
Temperature : Around 450°C
Catalysts : Iron [III] oxide [Fe2O3], promoter chromic oxide [Cr2O3]
Process : Excess steam is mixed with water gas, passed over a catalyst at elevated temperatures.
[CO is converted to CO2 with a further yield of hydrogen.]
3. Separation of carbon dioxide [CO2] and carbon monoxide from the above mixture
(a) CO2 is removed by dissolving mixture in water under pressure [30 atmospheres], or caustic potash solution to dissolve CO2.
2KOH + CO2 ⟶ K2CO3 + H2O
(b) CO is removed by dissolving mixture in ammoniacal cuprous chloride solution.
CuCl + CO + 2H2O ⟶ CuCl.CO.2H2O.
Thus, hydrogen gas is left behind.
Look at the following figure and answer the questions that follow:

(a) Which gas is prepared by this method marked as A.
(b) Name this method of collection. Why is this method used?
(c) Why is nitric acid not used as a reactant in the above method?
(d) Conc. H2SO4 is a good drying agent. However, it is not used here. Why?
Answer
(a) Hydrogen
(b) Downward displacement of water.
This method is used as:
- Hydrogen is virtually insoluble in water (20 ml of hydrogen dissolves in 1 litre of water under normal conditions)
- Hydrogen forms an explosive mixture with air and therefore, cannot be collected by downward displacement of air even though it is lighter than air.
(c) Nitric acid, even in its dilute form, is not used in the preparation of hydrogen from metals because it is a powerful oxidizing agent, and the oxygen formed due to its decomposition oxidizes the hydrogen to give water, thus defeating the purpose of the reaction.
3Zn + 8HNO3 ⟶ 3Zn(NO3)2 + 4H2O + 2NO↑
(d) Conc. H2SO4 is a good drying agent. However, it is not used to dry hydrogen as it reacts with hydrogen, thus defeating the purpose of the reaction.
H2SO4 + H2 ⟶ 2H2O + SO2
Identify the gas evolved in the figure shown below.
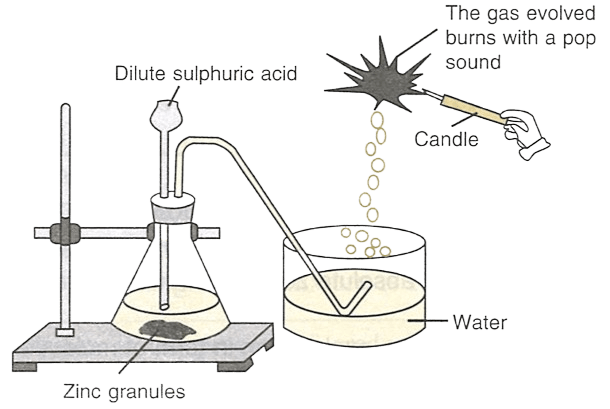
What would happen if the following changes are made:
(a) In place of zinc granules, same amount of zinc dust is taken in the test tube.
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) Sodium hydroxide is taken in place of dilute sulphuric acid and it is heated.
Answer
Gas evolved is Hydrogen.
(a) The reaction and the evolution of hydrogen gas will proceed more quickly if the same quantity of zinc dust is placed in the test tube. This is because the surface area of zinc dust is greater than that of zinc granules.
(b) When dilute hydrochloric acid is used in place of sulphuric acid, same amount of gas will evolve.
Zn + 2HCl (dil.) ⟶ ZnCl2 + H2 ↑
(c) Zinc reacts with hot concentrated alkalis like sodium hydroxide to form hydrogen and a soluble salt.
Zn + 2NaOH ⟶ Na2ZnO2 + H2 ↑
A metal is treated with dil. H2SO4. The gas evolved is collected by the method shown in the figure given below.
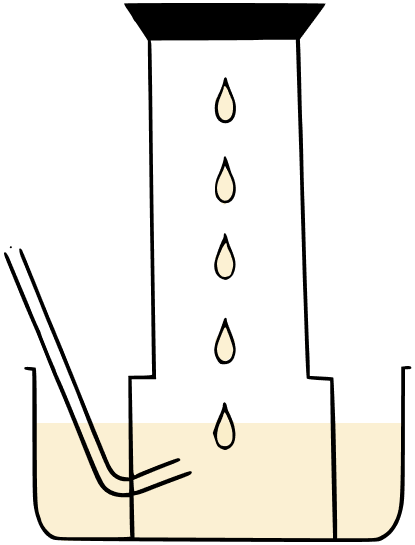
Answer the following:
(a) Name the gas.
(b) Name the method of collection of the gas.
(c) Is the gas soluble or insoluble in water?
(d) Is the gas lighter or heavier than air?
(e) Is the gas combustible or a supporter of combustion?
Answer
(a) Hydrogen.
(b) Downward displacement of water.
(c) Hydrogen gas is virtually insoluble in water.
(d) Hydrogen gas is lighter than air.
(e) Hydrogen gas is combustible but does not support combustion.