Name a positive non-metallic radical which is basic in nature.
Answer
Ammonium / NH4+
Reason — Ammonium / NH4+ is a weak base containing a non-metallic nitrogen radical.
How many electrons are present in one molecule of CH4?
Answer
10 electrons
Reason — carbon - 6 electrons, Hydrogen - 1 electron
There are four hydrogen atoms, so 4 electrons.
∴ Total number of electrons = 6 + 4 = 10 electrons.
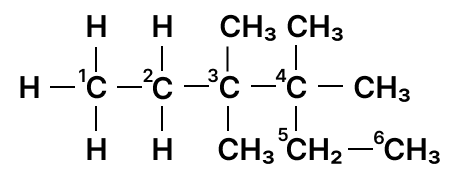
Identify the longest carbon chain and mention the number of carbons present in it.
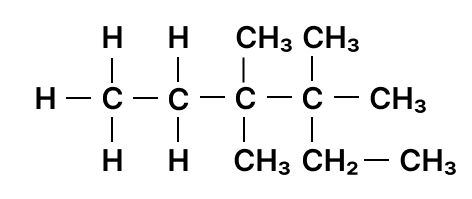
Answer
6
Reason — The below figure shows the longest carbon chain numbered:
Gas M occupies a volume of 1000 c.c and contains X molecules. How many molecules will be present in gas N occupying a volume of 250 c.c?
Answer
Reason — Let molecules in gas N be Y.
Hence, gas N contains molecules.
Element X belongs to period 2 and group 1 of the periodic table. State the formula of the chloride of the element X.
Answer
XCl
Reason — X is group 1 metal, it loses one electron to attain stability. Chlorine forms a Cl- ion. Both the ions combine together to form XCl.
Anurag added dilute H2SO4 to a given sample X and heated the mixture. He observed that a gas was liberated which had a foul smell of rotten eggs and it turned moist lead acetate paper silvery black. Name the gas evolved in the above case.
Answer
Hydrogen sulphide gas
Reason — When dilute H2SO4 is added to a given sample X and heated, it produces H2S gas, which had a foul smell of rotten eggs.
Metal sulphide + H2SO4 (dil.) ⟶ Metal salt + H2S
H2S turns moist lead acetate paper black.
H2S + Pb(CH3COO)2 ⟶ PbS(Black) + 2CH3COOH
Name the alkyl component of acetic acid.
Answer
-CH3 (methyl)
Reason — Acetic acid consists of a methyl group -CH3 and a carboxylic acid group -COOH. So, in acetic acid, the alkyl part is methyl group -CH3.
28g of nitrogen and 44g of carbon dioxide at the same conditions of temperature and pressure occupy the same amount of space. What term describes such space occupied by any gas?
Answer
Molar volume
Reason — When 28 g of nitrogen (N2) and 44 g of carbon dioxide (CO2) occupy the same volume, they are both 1 mole each, since:
Molar mass of N2 = 28 g/mol
Molar mass of CO2 = 44 g/mol
Hence, the space occupied is called the molar volume of a gas.
When copper reacts with a hot dilute solution, reddish-brown fumes are observed. Another compound, P, having the same anion that is present in the hot solution on heating, melts into a colourless liquid, releasing only oxygen gas without any coloured fumes. Identify P.
Answer
Sodium nitrate / Potassium nitrate
Reason — When copper reacts with a hot dilute solution, reddish-brown fumes of NO2 are observed.
Cu + 4HNO3 [dil.] ⟶ Cu(NO3)2 + 2H2O + 2NO2
Another compound, P, either Sodium nitrate or Potassium nitrate having the same anion NO3-, that is present in the hot solution, on heating, melts into a colourless liquid, releasing only oxygen gas without any coloured fumes.
↑
↑
Calcite (CaCO3), a sedimentary rock, is found most abundantly in many geological environments. It has a perfect cleavage in 3 directions, which makes it the most difficult rock to cut, and moreover, the labour of cutting calcite is also very high. What term related to metallurgy will suitably describe Calcite in the context of extracting calcium from calcite?
Answer
Mineral
Reason — Calcite (CaCO3) is a naturally occurring carbonate mineral. It serves as a source of calcium