Which gas decolourises potassium permanganate (KMnO4) solution?
- Sulphur dioxide
- Ammonia
- Hydrogen chloride
- Carbon dioxide
Answer
Sulphur dioxide
Reason — Sulphur dioxide gas turns acidified potassium permanganate from pink to clear colourless.
2KMnO4 + 2H2O + 5SO4 ⟶ K2SO4 + MnSO4 + H2SO4
Which formula represents a saturated hydrocarbon?
- C4H8
- C5H12
- C4H6
- C5H10
Answer
C5H12
Reason — C5H12 is an alkane that represents a saturated hydrocarbon. It is a simplest open chain hydrocarbon represented by the formula CnH2n+2.
The metal whose oxide can be reduced by common reducing agents:
- Copper
- Sodium
- Aluminium
- Potassium
Answer
Copper
Reason — Copper lies below hydrogen in the reactivity (activity) series, so its oxide is not very stable. Therefore, common chemical reducing agents such as carbon, carbon monoxide, or hydrogen can readily reduce copper(II) oxide to metallic copper, e.g.
CuO + H2 → Cu + H2O
In contrast, the oxides of sodium, aluminium and potassium are extremely stable because those metals are highly reactive and have a very strong affinity for oxygen. Their oxides can be reduced only by electrolysis and not by ordinary chemical reducing agents.
An organic compound has a vapour density of 22. The molecular formula of the organic compound is: [Atomic weight: C = 12, H = 1]
- CH4
- C2H4
- C2H6
- C3H8
Answer
C3H8
Reason
Given,
Vapour density of organic compound = 22
Molecular weight = 2 x V.D. = 2 x 22 = 44
| Compound | Molecular mass |
|---|---|
| 1. CH4 | 16 |
| 2. C2H4 | 28 |
| 3. C2H6 | 30 |
| 4. C3H8 | 44 |
Hence, C3H8 is a organic compound that has the molecular mass of 44.
In the reaction given below sulphuric acid acts as a/an:
S + 2H2SO4 ⟶ 3SO2 + 2H2O
- Non-volatile acid
- Dibasic acid
- Oxidising agent
- Reducing agent
Answer
Oxidising agent
Reason — Oxidising agents are substances which add oxygen atoms to others compounds or remove hydrogen atoms from others compounds. Sulphuric acid accepts electrons and donates oxygen atoms to sulphur, leading to the formation of SO2 and water (H2O). Thus, sulphuric acid acts as an oxidising agent.
Assertion (A): The tendency of losing electrons increases down the Group.
Reason (R): The most reactive metal is placed at the top of Group 1.
- Both (A) and (R) are true, and (R) is the correct explanation of (A).
- Both (A) and (R) are true, and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
Answer
(A) is true but (R) is false.
Reason — As we move down any group in the periodic table, atomic size increases and the outermost electron is held less tightly by the nucleus. Therefore the loss of this electron becomes easier, so the tendency to lose electrons increases; Assertion (A) is true. In group 1 the reactivity of metals increases down the group for the same reason. Consequently, the most reactive metal is found at the bottom, not at the top; Reason (R) is false.
The ore that can be concentrated by using magnetic separation:
- Corundum
- Haematite
- Calamine
- Bauxite
Answer
Haematite
Reason — Magnetic separation works when either the ore or the gangue shows magnetic behaviour. Haematite, an iron ore, is weakly magnetic, whereas its common impurity (silica) is non-magnetic. When the crushed mixture is passed over a high-intensity magnetic separator, the haematite particles are attracted and collected separately from the non-magnetic gangue, concentrating the ore.
The diagram given below shows the bonding in the covalent molecule AB2.
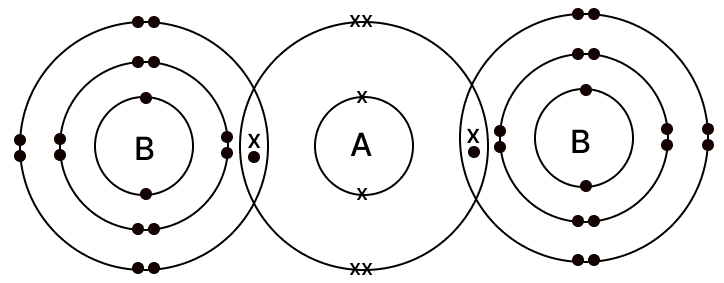
Which option represents the correct electronic configuration of atoms A and B before combining together to form the above molecule?
| A | B | |
|---|---|---|
| 1 | 2, 4 | 2, 8, 6 |
| 2 | 2, 4 | 2, 8, 7 |
| 3 | 2, 8 | 2, 8, 8 |
| 4 | 2, 6 | 2, 8, 7 |
Answer
| 4 | 2, 6 | 2, 8, 7 |
Reason
- Atom A has six valence electrons (configuration 2, 6) and therefore needs two more electrons to complete its octet.
- Each atom B has seven valence electrons (configuration 2, 8, 7) and needs one additional electron to attain a stable, noble-gas configuration.
- In the AB2 molecule, atom A forms a single covalent bond with each of two B atoms. Through this sharing, atom A gains two electrons (one from each B), while each B gains one electron from A, so all three atoms achieve an octet.
Which of the following options has all the compounds which are members of the same homologous series?
- CH4, C2H6, C3H8
- CH4, C2H6, C3H6
- C3H4, C3H6, C3H8
- C2H4, C3H6, C4H10
Answer
CH4, C2H6, C3H8
Reason — These three molecules are consecutive members of the alkane series, which has the general formula CnH2n+2. Each successive member differs from the previous one by a CH2 group, satisfying the defining characteristic of a homologous series.
Assertion (A): In the Contact Process SO3 gas is not directly dissolved in water to obtain sulphuric acid.
Reason (R): Dense fog or misty droplets of sulphuric acid are formed which is difficult to condense.
- Both (A) and (R) are true, and (R) is the correct explanation of (A).
- Both (A) and (R) are true, and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
Answer
Both (A) and (R) are true, and (R) is the correct explanation of (A).
Reason — When sulphur trioxide is brought into direct contact with water,
SO3(g) + H2O(l) ⟶ H2SO4(l)
the reaction is highly exothermic. The heat released vaporises some of the acid and produces a dense fog of minute H2SO4 droplets, which are difficult to condense and collect. Therefore, in the Contact Process the SO3 is first absorbed in concentrated sulphuric acid to form oleum, which is later diluted with water to give the required concentration of H2SO4.
Given below are four ions:
Cl-, Li+, Al3+, K+
Identify the pair of ions which have the same electronic configuration.
[Atomic number: Cl = 17, Li = 3, Al = 13, K = 19]
- Cl- & Li+
- Al3+ & K+
- Cl- & K+
- Li+ & K+
Answer
Cl- & K+
Reason
- Chlorine has atomic number 17. Its neutral atom contains 17 electrons with the configuration 2, 8, 7. When it gains one electron to form Cl⁻, it has 18 electrons, giving the configuration 2, 8, 8 (the same as argon).
- Potassium has atomic number 19. A neutral K atom has the configuration 2, 8, 8, 1. When it loses one electron to form K⁺, it is left with 18 electrons, also giving the configuration 2, 8, 8.
Because both Cl⁻ and K⁺ possess 18 electrons and share the electron configuration 2, 8, 8, they form the required pair with identical electronic configurations.
Which pair of reactants can be best used to produce lead (II) sulphate?
- Sulphuric acid + Lead
- Sulphuric acid + Lead hydroxide
- Sodium sulphate + Lead nitrate
- Potassium sulphate + Lead oxide
Answer
Sodium sulphate + Lead nitrate
Reason — Both sodium sulphate (Na2SO4) and lead(II) nitrate [Pb(NO3)2] are readily soluble in water. When their aqueous solutions are mixed, a double-decomposition reaction occurs, forming insoluble lead(II) sulphate (PbSO4) as a white precipitate:
Pb(NO3)2(aq) + Na2SO4(aq) ⟶ PbSO4(s) ↓ + 2NaNO3(aq)
Because the desired product precipitates out directly and the by-product (sodium nitrate) remains in solution, this pair of reactants is the most convenient for preparing pure lead(II) sulphate.
Aqueous copper (II) sulphate is electrolysed using copper electrodes. Which statement about the electrolysis is not correct?
- An oxidation reaction occurs at the positive electrode.
- The current is carried through the electrolyte by ions.
- The positive electrode loses mass.
- The number of copper (II) ions in the electrolyte decreases.
Answer
The number of copper (II) ions in the electrolyte decreases.
Reason — Cu2+ ions deposited at the cathode are replaced by Cu2+ ions produced at the anode, so the overall Cu2+ concentration stays constant.
X, Y & Z are three metallic atoms in successive order belonging to the same group such that atomic radii of ‘X’ is the smallest. Which of the three atoms is the best reducing agent?
- X
- Y
- Z
- All three have the same reducing power.
Answer
Z
Reason — The greater the tendency to lose electrons, the greater the metallic character and the stronger the reducing power of a metal. As we move down a group, atomic size increases and ionisation energy decreases, so the tendency to lose electrons and thus the reducing power also increases. Therefore, of the three atoms, Z (the lowest member of the group) is the best reducing agent.
40 cm3 of methane (CH4) is reacted with 60 cm3 of oxygen. The equation for the reaction is given below:
CH4 + 2O2 ⟶ CO2 + 2H2O
All volumes are measured at room temperature. What is the total volume of the gases remaining at the end of the reaction?
- 60 cm3
- 40 cm3
- 45 cm3
- 50 cm3
Answer
40 cm3
Reason — From the equation :
CH4 + 2O2 ⟶ CO2 + 2H2O
1 volume of CH4 reacts with 2 volumes of O2
For 40 cm3 of CH4 the required O2 is 40 cm3 x 2 = 80 cm3
Since only 60 cm3 of O2 is available.
The amount of CH4 that reacts with 60 cm3 of O2 is = = 30 cm3
∴ The excess CH4 remaining is 40 cm3 - 30 cm3 = 10 cm3
From the balanced equation, 2 volumes of O2 produce 1 volume of CO2.
∴ The volume of CO2 produced from 60 cm of O2 is = 30 cm3
The remaining gases are excess CH4 and produced CO2.
∴ Total volume = 10 cm3 (excess CH4) + 30 cm3 (CO2) = 40 cm3
A student was instructed by the teacher to prepare and collect ammonia gas in the laboratory by using aluminium nitride. The student had set up the apparatus as shown in the diagram below. Study the given diagram and answer the following questions:
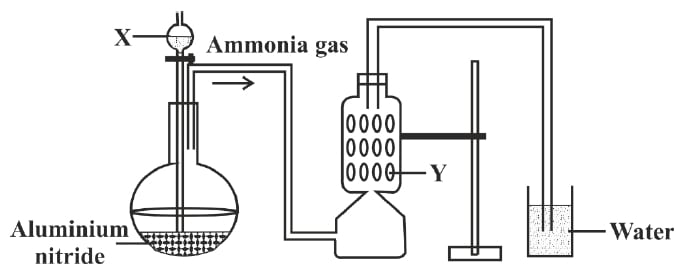
(a) Name the substance X added through the thistle funnel by the student.
(b) Write a balanced equation for the reaction occurring between Aluminium nitride and substance X.
(c) Identify the substance Y.
(d) State the function of Y.
(e) Why could the student not collect ammonia gas at the end of the experiment?
Answer
(a) X — Water
(b) AlN + 3H2O ⟶ Al(OH)3 + NH3↑
(c) Y — Quicklime (CaO)
(d) Substance Y — Quicklime (CaO) is used as a drying agent
(e) Ammonia gas is highly soluble in water so it cannot be collected over water hence it is collected by the downward displacement of air.
State the terms for the following:
(a) Undistilled alcohol containing a large amount of methanol.
(b) A salt formed by the partial replacement of the hydroxyl group of a di-acidic or a tri-acidic base by an acid radical.
(c) Organic compounds having the same molecular formula but different structural formula.
(d) The tendency of an atom to attract the shared pair of electrons towards itself when combined in a compound.
(e) The type of covalent bond in which electrons are shared unequally between the combining atoms.
Answer
(a) Spurious alcohol
(b) Basic salt
(c) Isomers
(d) Electronegativity
(e) Polar covalent bond.
Complete the following sentences by choosing the correct word(s) from the brackets:
(a) ............... solution forms a coloured precipitate with ammonium hydroxide which is soluble in excess of ammonium hydroxide. [Ferrous chloride / Copper nitrate]
(b) Zinc blende is converted to zinc oxide by ................ [Calcination / Roasting]
(c) ............... conducts electricity by the movement of ions. [Molten iron / Molten sodium chloride]
(d) The reaction that takes place at the anode during the electrolysis of aqueous Sodium argentocyanide with silver electrodes is ................ [Ag ⟶ Ag+ + e-/ Ag+ + e- ⟶ Ag]
(e) The salt formed when ZnO reacts with hot concentrated NaOH is ............... . [sodium zincate / zinc hydroxide]
Answer
(a) Copper nitrate solution forms a coloured precipitate with ammonium hydroxide which is soluble in excess of ammonium hydroxide.
(b) Zinc blende is converted to zinc oxide by roasting.
(c) Molten sodium chloride conducts electricity by the movement of ions.
(d) The reaction that takes place at the anode during the electrolysis of aqueous Sodium argentocyanide with silver electrodes is Ag ⟶ Ag+ + e-
(e) The salt formed when ZnO reacts with hot concentrated NaOH is sodium zincate.
Match the Column A with Column B:
| Column A | Column B |
|---|---|
| (a) N2 + 3H2 ⇌ 2NH3 | 1. Vanadium Pentoxide |
| (b) 4NH3 + 5O2 ⟶ 4NO + 6H2O | 2. Nickel |
| (c) 2SO2 + O2 ⇌ 2SO3 | 3. Iron |
| (d) C2H4 + H2 ⟶ C2H6 | 4. Concentrated Sulphuric acid |
| (e) CuSO4.5H2O ⟶ CuSO4 + 5H2O | 5. Platinum |
Answer
| Column A | Column B |
|---|---|
| (a) N2 + 3H2 ⇌ 2NH3 | 3. Iron |
| (b) 4NH3 + 5O2 ⟶ 4NO + 6H2O | 5. Platinum |
| (c) 2SO2 + O2 ⇌ 2SO3 | 1. Vanadium Pentoxide |
| (d) C2H4 + H2 ⟶ C2H6 | 2. Nickel |
| (e) CuSO4.5H2O ⟶ CuSO4 + 5H2O | 4. Concentrated Sulphuric acid |
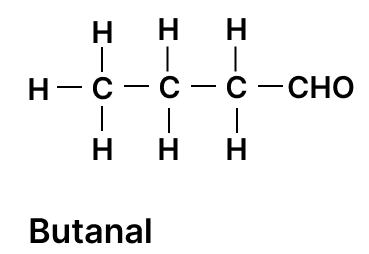
(a) Draw the structural diagram for the following organic compounds:
- 2-methyl propene
- butanal
(b) Give IUPAC name for the following organic compounds:
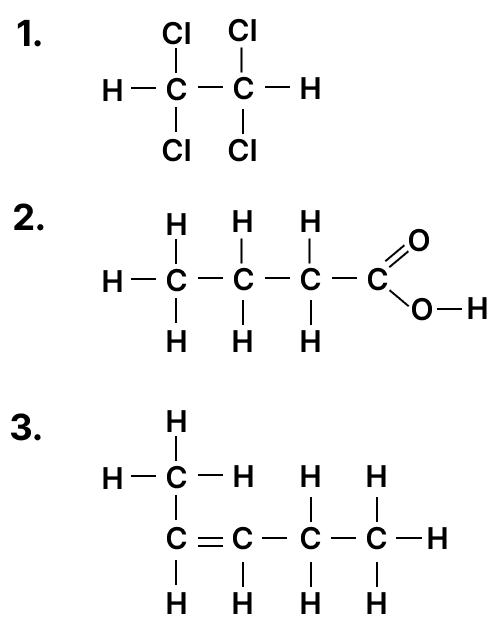
Answer
(a)
- 2-methyl propene

- butanal
(b) IUPAC names
- 1,1,2,2-Tetrachloroethane
- Butanoic acid
- Pent-2-ene
The atomic number of two atoms ‘X’ and ‘Y’ are 14 and 8 respectively.
State:
(a) the period to which ‘X’ belongs.
(b) the formula of the compound formed between ‘X’ and ‘Y’.
(Do not identify X and Y)
Answer
(a) The atomic number of X is 14
Electronic configuration ⟶ 2, 8, 4
Number of electron shells = 3, hence X belongs to period 3
(b) The atomic number of Y is 8
Electronic configuration ⟶ 2, 6
X has a valency of 4 and Y has valency of 2
∴ Simplified formula of the compound is XY2
Justify the following statements:
(a) Anode is known as the oxidizing electrode.
(b) Graphite electrodes are preferred in the electrolysis of molten lead bromide.
Answer
(a) Oxidation is the loss of electrons. In an electrolytic cell the anode is the positive electrode that attracts anions. These anions give up electrons to the anode, so oxidation takes place there. Because the anode accepts electrons and thereby causes the ions to be oxidised, it is called the oxidising electrode.
(b) As graphite is unaffected by the reactive bromine vapours released at the anode hence, a graphite anode is preferred to other inert electrodes like platinum during the electrolysis of molten lead bromide.
The reaction between concentrated sulphuric acid and magnesium can be represented by the equation given below:
Mg + 2H2SO4 ⟶ MgSO4 + 2H2O + SO2
If 60 g of magnesium is used in the reaction, calculate the following:
(a) The mass of sulphuric acid needed for the reaction.
(b) The volume of sulphur dioxide gas liberated at S.T.P.
[Atomic weight: Mg=24, H=1, S=32, O=16]
Answer
(a) 24 g of Mg reacts with 196 g of H2SO4
∴ 60 g of Mg reacts with x 60 = 490 g of H2SO4
Hence, 490 g of H2SO4 is required.
(b) 24 g of Mg gives 22.4 L volume of SO2
∴ 60 g of Mg gives x 60 = 56 L
Hence, the volume of sulphur dioxide gas liberated at S.T.P. is 56 L.
Thus, 490 g of sulphuric acid are needed and 56 L of SO2 will be liberated at S.T.P.
Give one significant observation when:
(a) a solution of barium chloride is added to zinc sulphate solution.
(b) lead nitrate is heated in a test tube.
(c) chlorine gas is passed over moist starch iodide paper.
Answer
(a) When barium chloride solution is added to a solution of Zinc sulphate, white ppt. of barium sulphate is obtained which is insoluble in dil. HCl or nitric acid.
ZnSO4 + BaCl2 ⟶ BaSO4 ↓ [white ppt.] + ZnCl2
(b) When lead nitrate is heated in a test tube, brown fumes of nitrogen dioxide (NO2) are evolved and a yellow residue of lead(II) oxide remains.
2Pb(NO3)2 2PbO + 4NO2 + O2
(c) The moist starch-iodide paper turns blue-black because chlorine liberates iodine from iodide.
Cl2 + 2KI ⟶ 2KCl + I2
A gas cylinder can hold 150 g of hydrogen under certain conditions of temperature and pressure. If an identical cylinder with the same capacity can hold 450 g of gas ‘G’ under the same conditions of temperature and pressure, find:
(a) the vapour density of the gas ‘G’.
(b) the molecular weight of gas ‘G’.
Answer
(a ) V.D. = = = 3
(b) Molecular weight = 2 x V.D. = 2 x 3 = 6 a.m.u.
Complete and balance the following equations:
(a) CH3COONa + NaOH
(b) CH3COOH + Mg ⟶
Answer
(a) CH3COONa + NaOH CH4 + Na2CO3
(b) 2CH3COOH + Mg ⟶ (CH3COO)2Mg + H2 ↑
Name the gas produced during each of the following reactions:
(a) When copper is treated with hot, concentrated nitric acid.
(b) When ammonia is burnt in an atmosphere of oxygen.
(c) When ferrous sulphide reacts with dilute hydrochloric acid.
Answer
(a) Nitrogen dioxide (NO2)
(b) Nitrogen (N2)
(c) Hydrogen sulphide (H2S)
Study the table given below. Use only the letters given in the table to answer the questions. Do not identify the elements.
| IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
|---|---|---|---|---|---|---|---|
| E | J | Q | |||||
| L | G | ||||||
| M | D | P | |||||
| N |
(a) State the valency of element ‘G’.
(b) Which element can exhibit catenation?
(c) Write the formula of the compound formed between ‘M’ and ‘P’.
Answer
(a) Element G
Reason — Element G belongs to group VA (group 15). Group 15 elements have 5 valence electrons in their outermost shell they can gain 3 electrons to complete the octet hence, the valency will be 3.
(b) Element E
Reason — Catenation (ability to form long chains of its own atoms) is strongest in the group IVA (14) element shown, i.e., E.
(c) Element m
Reason — M is in group IIA they have 2 valence electrons and forms M2+ and P is in group VIIA have 7 valence electrons and forms P-, so one molecule of M combines with two molecules of P. Hence, the neutral formula is MP2.
Given below are two sets of elements from two different periods. Name the element with the highest ionisation potential in each of the following sets.
(a) Al, Cl, Mg
(b) Ne, O, F
Answer
(a) Cl
(b) Ne
Reason
(a) Cl — Among the three elements, Cl is the rightmost in period 3. As ionisation energy increases when moving across a period from left to right hence ionisation energy is highest for Cl.
(b) Ne — It is the rightmost element in period 2 and an inert gas. Hence it has the highest ionisation potential.
Ammonia gas is passed over heated copper (II) oxide in a combustion tube:
(a) Name the gas evolved.
(b) What will be the colour of the residue that is left in the combustion tube at the end of the reaction?
Answer
(a) Nitrogen (N2)
(b) Reddish-brown
Reason
(a) When ammonia gas is passed over heated copper (II) oxide in a combustion tube nitrogen gas is evolved.
2NH3 + 3CuO ⟶ 3Cu + 3H2O + N2 [g]
(b) The residue is reddish-brown copper metal.
Give balanced equations for the following:
(a) Action of dilute hydrochloric acid on ammonium carbonate.
(b) Oxidation of sulphur with hot concentrated nitric acid.
(c) Reaction of concentrated sulphuric acid with carbon.
Answer
(a) (NH4)2CO3 + 2HCl ⟶ 2NH4Cl + H2O + CO2
(b) S + 6HNO3(conc, hot) ⟶ H2SO4 + 6NO2 + 2H2O
(c) C + 2H2SO4 [conc.] ⟶ CO2 + 2H2O + 2SO2
Rohit took two different salt solutions in test tubes C and D as shown in the figure below. He added dilute HCl to each of the two test tubes. The products formed in the test tubes C and D are silver chloride and lead chloride respectively.
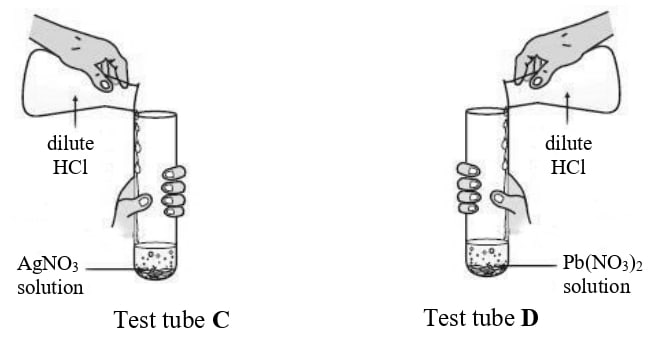
State:
(a) one common observation made by Rohit in both the reactions.
(b) the observations made by him on addition of excess of ammonium hydroxide to the products formed in:
- test tube C
- test tube D
Answer
(a) In each test tube a white precipitate is formed.
(b)
- In test tube C, the white precipitate of silver chloride dissolves in excess ammonium hydroxide, giving a clear solution.
- In test tube D, the white precipitate of lead(II) chloride does not dissolve in excess ammonium hydroxide and remains unchanged.
Reason
(a) When dilute hydrochloric acid is added to silver nitrate (AgNO3) solution in test tube C, silver chloride (AgCl) is formed, which is a white precipitate. Similarly, when dilute HCl is added to lead(II) nitrate (Pb(NO3)2)solution in test tube D, lead(II) chloride (PbCl2) is formed, which is also a white precipitate. Therefore, a common observation is the formation of a white precipitate in both test tubes.
(b) In test tube C, the white precipitate of silver chloride dissolves in excess ammonium hydroxide whereas, in test tube D, the white precipitate of lead chloride does not dissolve in excess ammonium hydroxide.
Given below is a diagram showing the placement of five different oxides. With respect to the given diagram answer the following questions:
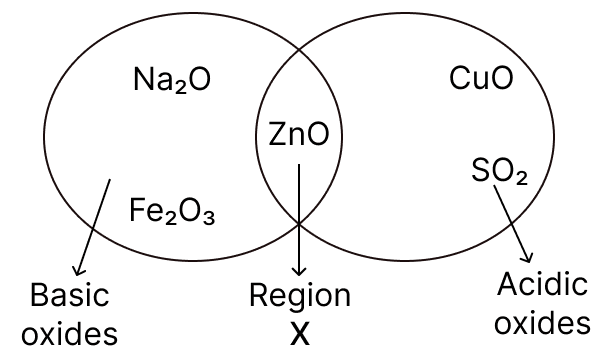
(a) Name the type of oxide represented in region X in the diagram.
(b) Identify the oxide which has been incorrectly placed in the above diagram.
(c) Name the oxide from the above diagram which will form an alkali when dissolved in water.
Answer
(a) Amphoteric oxides
(b) Copper(II) oxide is placed incorrectly. It is a basic oxide, but it is shown under acidic categories.
(c) Sodium oxide (Na2O) is the oxide that will form an alkali when dissolved in water:
Na2O + H2O ⟶ 2NaOH
Given below are organic compounds labelled A to F. Answer the questions that follow:
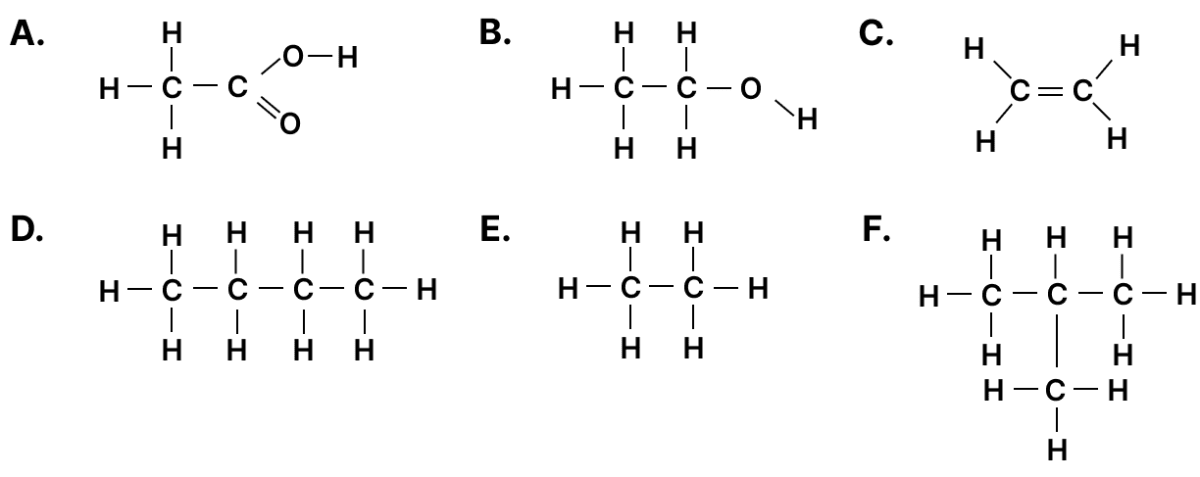
(a) Which compound forms a single product with bromine?
(b) Which two compounds have the same molecular formula?
(c) Which two compounds will react together in the presence of concentrated H2SO4 to form a product with a fruity smell?
Answer
(a) C
(b) D and F
(c) A and B
Reason
(a) C reacts with bromine to give a single product 1,2-dibromoethane
(b) D is a straight-chain butane and F is a branched isobutane or 2-methylpropane, they have the same molecular formula C4H10 and are structural isomers of each other.
(c) A is a carboxylic acid (CH3COOH) and B is an alcohol (CH3CH2OH). In presence of concentrated H2SO4 they undergo esterification to give ethyl ethanoate (ethyl acetate), which has a fruity smell.
An organic compound ‘X’ contains carbon, oxygen and hydrogen only. The percentage of carbon and hydrogen are 47.4% and 10.5% respectively. The relative molecular mass of ‘X’ is 76. Find the empirical formula and the molecular formula of ‘X’.
[Atomic weight: C = 12, O = 16, H = 1]
Answer
| Element | % composition | At. wt. | Relative no. of atoms | Simplest ratio |
|---|---|---|---|---|
| Carbon | 47.4 | 12 | = 3.95 | = |
| Hydrogen | 10.5 | 1 | = 10.5 | = 4 |
| Oxygen | 42.1 | 16 | = 2.63 | = 1 |
Simplest ratio of whole numbers = C : H : O = : 4 : 1 = 3 : 8 : 2
Hence, empirical formula is C3H8O2
Empirical formula weight = (3 x 12) + (8 x 1) + (2 x 16)= 36 + 8 + 32 = 76
Empirical formula weight = Relative molecular mass = 76
So, molecular formula = C3H8O2
Seema added a few pieces of copper turnings to a test tube containing concentrated acid P and she noticed that a reddish-brown gas evolved.
(a) Name the acid P used by Seema.
(b) Write a balanced chemical equation for the reaction that took place.
Answer
(a) The acid P is concentrated nitric acid (HNO3).
(b) Cu + 4HNO3 ⟶ Cu(NO3)2 + 2H2O + 2NO2
Answer the following questions with reference to the concentration of bauxite ore.
(a) Name the process used to concentrate the ore.
(b) Give a balanced chemical equation for the conversion of aluminium hydroxide to pure alumina.
Answer
(a) Bayer's process is used to concentrate bauxite ore to alumina.
(b)
Draw the dot and cross structure of the following:
(a) An ionic compound formed when Mg reacts with the dilute HCl.
(b) A covalent compound formed when H2 reacts with Cl2.
(c) The positive ion produced when ammonia gas is dissolved in water.
[Atomic number: Mg = 12, Cl = 17, H = 1, N = 7]
Answer
(a) When Mg reacts with the dilute HCl, MgCl2 formed.

(b) Covalent compound formed when H2 reacts with Cl2 is HCl.

(c) The positive ion produced when ammonia gas is dissolved in water is ammonium ion.
![Draw an electron dot diagram to show the formation of ammonium ion [N = 7, H = 1]. Chemical Bonding, Simplified Chemistry Dalal Solutions ICSE Class 10](https://cdn1.knowledgeboat.com/img/scd10/ammonium-ion-electron-dot-diagram-simplified-chemistry-icse-class-10-1200x305.png)
Acidulated water is electrolysed using platinum electrodes. Answer the following questions:
(a) Why is dilute sulphuric acid added to water?
(b) Write the reaction taking place at the cathode.
(c) What is the observation at the anode?
Answer
(a) Water in pure state consists almost entirely of molecules. It is a polar covalent compound and can form ions when traces of dilute sulphuric acid is added.
(b) 4H+ + 4e- ⟶ 4H
2H + 2H ⟶ 2H2
(c) At the anode (positive electrode) hydroxide ions are preferentially discharged, producing oxygen gas:
4OH- - 4e- ⟶ 4OH
4OH ⟶ 2H2O + O2
(a) State Avogadro’s Law.
(b) Define Co-ordinate bond.
Answer
(a) Avogadro’s Law — Under the same conditions of temperature and pressure equal volumes of all gases contain the same number of molecules.
(b) Co-ordinate bond — The bond formed between two atoms by sharing a pair of electrons, provided entirely by one of the combining atoms but shared by both is called a coordinate bond.
Differentiate between the following pairs of compounds using the reagent given in the bracket:
(a) Ammonium chloride and Sodium chloride (using an alkali)
(b) Zinc Nitrate solution and Calcium Nitrate solution (using excess sodium hydroxide solution)
Answer
(a) When ammonium chloride is reacted with an alkali like NaOH, the colourless gas ammonia having a sharp pungent characteristic smell is evolved. Whereas, sodium chloride (NaCl) does not show any reaction with alkali.
NH4Cl + NaOH ⟶ NH3 ↑ + NaCl + H2O
NaCl + NaOH ⟶ No reaction
(b) Add sodium hydroxide (NaOH) solution first drop by drop and then in excess.
- Zinc Nitrate solution: A white gelatinous precipitate of zinc hydroxide forms, which dissolves in excess NaOH to give a clear solution.
- Calcium Nitrate solution: A white precipitate of calcium hydroxide forms and remains insoluble even in excess NaOH.
You are provided with some compounds in the box.
| PbO | CH4 | PbO2 | CO2 | |||
| HCl | NCl3 | SO2 |
Choose the most appropriate compound which fits the descriptions (a) to (c) given below:
(a) A colourless gas which turns acidified K2Cr2O7 from orange to green.
(b) A yellow explosive oily liquid formed when excess chlorine gas reacts with ammonia gas.
(c) A yellow metallic oxide formed on thermal decomposition of PbCO3.
Answer
(a) SO2
Reason — Sulphur dioxide is a colourless gas. It is a reducing agent and turns acidified potassium dichromate solution from orange to green.
(b) NCl3
Reason — Nitrogen trichloride is a yellow explosive oily liquid formed when excess chlorine reacts with ammonia.
NH3 + 3Cl2 ⟶ NCl3 + 3HCl
(c) PbO
Reason — Lead(II) oxide is yellow and is produced when lead carbonate (PbCO3) undergoes thermal decomposition.
PbCO3 ⟶ PbO + CO2
P, Q, R and S are the different methods of preparation of salts.
P – Simple displacement
Q – Neutralisation by titration
R – Precipitation
S – Direct combination
Choose the most appropriate method to prepare the following salts:
(a) PbCl2
(b) FeCl3
(c) Na2SO4
Answer
(a) PbCl2 — R: Precipitation
(b) FeCl3 — S: Direct combination
(c) Na2SO4 — Q: Neutralisation by titration