Read the statements given below:
I — Copper is a component in the alloy that is used to make medals.
II — Aluminium is used in making the alloy stainless steel.
III — Copper is a common component of both duralumin and brass.
Which of the statements are correct?
- I & II
- I & III
- II & III
- I, II & III
Answer
I & III
Reason — Copper is a main component of alloy bronze that is used to make medals and copper is also a main component of brass and duralumin whereas aluminium is not a part of stainless steel.
Sodium hydroxide can react with ............... acid to form an acid salt.
- Nitric acid
- Hydrochloric acid
- Acetic acid
- Sulphuric acid
Answer
Sulphuric acid
Reason — An acid salt is formed when a dibasic or tribasic acid is only partially neutralised by a base.
- Nitric acid (HNO3) and hydrochloric acid (HCl) are monobasic acids → they can form only normal salts, not acid salts.
- Acetic acid (CH3COOH) is also monobasic → only forms sodium acetate.
- Sulphuric acid (H2SO4) is dibasic → with sodium hydroxide (NaOH), it can form an acid salt, sodium hydrogen sulphate (NaHSO₄), on partial neutralisation.
So, sodium hydroxide reacts with sulphuric acid to form an acid salt.
NaOH + H2SO4 ⟶ NaHSO4 + H2O
How many moles are present in 10g of CaCO3? [Atomic weight: Ca = 40, C = 12, O = 16]
- 10 moles
- 1 mole
- 0.1 mole
- 0.11 mole
Answer
0.1 mole
Reason — Number of moles present in 10g of CaCO3
Molar mass of CaCO3 = 40 + 12 + 3(16) = 40 + 12 + 48 = 100 g
100 g of CaCO3 = 1 mole
∴ 10 g of CaCO3 =
= 0.1 mole
A white precipitate is formed when dilute hydrochloric acid reacts with ‘X’. The white precipitate is soluble in excess of NH4OH and insoluble in dilute HNO3. Identify ‘X’.
- AgNO3
- NH4Cl
- AgCl
- CaCl2
Answer
AgNO3
Reason — A white precipitate of AgCl is formed when dilute hydrochloric acid reacts with AgNO3. Silver chloride is a white precipitate that is soluble in excess of Ammonium hydroxide solution and insoluble in dilute HNO3.
AgNO3 + HCl ⟶ AgCl + HNO3
Assertion (A): In a solution containing equal concentration of Cu2+ ions and Ca2+ ions, Cu2+ ions will be discharged in preference to Ca2+ ions.
Reason (R): Ca2+ ions are placed above Cu2+ ions in the electrochemical series.
- Both (A) and (R) are true, and (R) is the correct explanation of (A).
- Both (A) and (R) are true, and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
Answer
Both (A) and (R) are true, and (R) is the correct explanation of (A).
Reason — Cu2+ ions will be discharged in preference to Ca2+ ions because Cu2+ ions are present lower than Ca2+ ions in the electrochemical series.
Elements lower in the series get discharged more easily at the cathode during electrolysis because their cations can easily gain electrons. Hence, the assertion (A) is true.
The Cu2+ ions will be discharged in preference to Ca2+ ions because Ca2+ ions are placed above Cu2+ ions in the electrochemical series. Hence, the reason (R) is true and it correctly explains assertion (A).
Assertion (A): Hydraulic washing is a method to separate impurities from the ore.
Reason (R): In Hydraulic washing, denser particles float and lighter particles settle down.
- Both (A) and (R) are true and (R) is the correct explanation of (A).
- Both (A) and (R) are true and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
Answer
(A) is true but (R) is false.
Reason — Hydraulic washing (gravity separation) is a method to separate impurities (gangue) from the ore. Hence, the assertion (A) is true.
During the hydraulic washing, denser particles settle at the bottom whereas lighter particles float on the surface. Hence, the reason (R) is false.
The oxide which reacts with both dilute hydrochloric acid and sodium hydroxide solution to form salt and water is:
- Basic oxide
- Amphoteric oxide
- Acidic oxide
- Neutral oxide
Answer
Amphoteric oxide
Reason — Amphoteric oxides can react with both acids and bases to form salt and water. For example: Al2O3
Which of the following will occupy the volume 2.8 litres at S.T.P.?
(Atomic weight: C = 12, O = 16, Cl = 35.5, S = 32)
- 2 moles of carbon dioxide
- 7.1 g of chlorine
- 8 g of sulphur dioxide
- 56 g of carbon monoxide
Answer
8 g of sulphur dioxide
Reason — One mole of any gas occupies 22.4 litres of volume at S.T.P.
The required moles of gas at S.T.P = = 0.125 mol
| Substance | Molar mass(g) | Given mass (moles/g) | No. of moles | Volume at STP (L) |
|---|---|---|---|---|
| 2 moles of carbon dioxide | 44 | 2 mol | 2 | 44.8 |
| 7.1 g of chlorine | 71 | 7.1 g | 0.10 | 2.24 |
| 8 g of sulphur dioxide | 64 | 8g | 0.125 | 2.8 |
| 56 g of carbon monoxide | 28 | 56g | 2.00 | 44.8 |
∴ 8 g of sulphur dioxide will occupy the volume 2.8 litres at S.T.P.
A salt solution which gives a reddish-brown precipitate with NaOH and a white precipitate with BaCl2 solution is:
- CuSO4
- Ca(NO3)2
- Fe2(SO4)3
- FeCl3
Answer
Fe2(SO4)3
Reason —
Reddish-brown ppt with NaOH indicates Fe3+ ions.
White ppt with BaCl2 indicates SO42- ions (forms insoluble BaSO4)
So the salt must contain Fe3+ and SO42-, which is Fe2(SO4)3.
An alkane with molecular mass 44 is:
- CH4
- C3H8
- C4H10
- C2H6
Answer
C3H8
Reason — Let's check the molecular masses:
- CH4 → 12 + 4×1 = 16
- C2H6 → 2×12 + 6×1 = 24 + 6 = 30
- C3H8 → 3×12 + 8×1 = 36 + 8 = 44
- C4H10 → 4×12 + 10×1 = 48 + 10 = 58
So, the alkane with molecular mass 44 is C3H8 (propane).
The gas evolved in the diagrammatic set up given below turns calcium hydroxide solution milky. The gas evolved is:
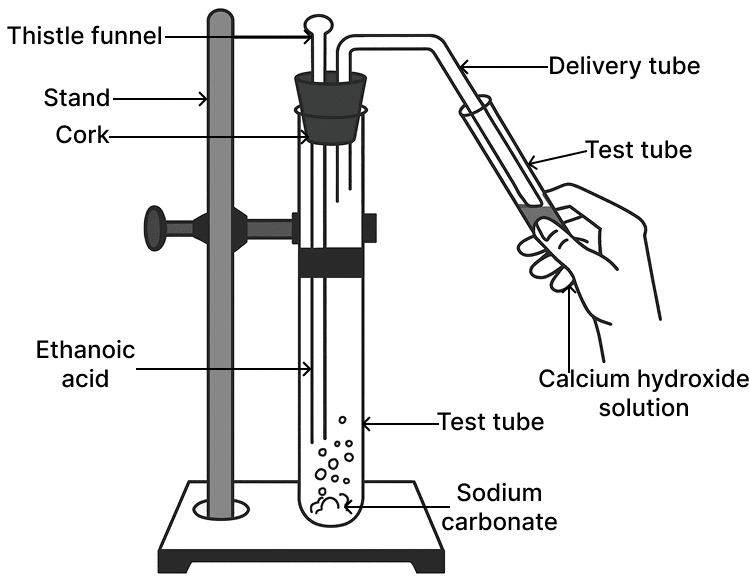
- CH4
- C2H6
- CO2
- SO2
Answer
CO2
Reason — The setup shows ethanoic acid reacting with sodium carbonate. This reaction produces carbon dioxide gas, which when passed through calcium hydroxide solution [lime water] turns it milky due to the formation of insoluble calcium carbonate:
Hence, the gas evolved is carbon dioxide (CO₂).
Which gas is evolved when ammonia gas is passed over buff yellow PbO?
- N2O
- NO
- N2
- NO2
Answer
N2
Reason — When ammonia gas is passed over buff yellow PbO, ammonia reduces heated lead monoxide to greyish metallic lead with the liberation of nitrogen gas.
2NH3 + 3PbO ⟶ 3Pb + 3H2O + N2 ↑
Three different solutions X (sodium chloride solution), Y (acetic acid) and Z (sugar solution) were used for electrolysis by a student. When the circuit was completed, he noticed that the bulb glowed in the electrolytic cell containing:
- X & Y
- Y & Z
- Z & X
- X, Y & Z
Answer
X & Y
Reason — X (NaCl solution) is an electrolyte, it contains free Na+ and Cl- ions, so it conducts electricity well.
Y (acetic acid) is a weak electrolyte, it partially ionises to give H+ and CH3COO- , so it can conduct where the bulb may glow dimmer than with a strong electrolyte. Whereas, Z (sugar solution) contains molecular sucrose which does not dissociate into ions.
An element X has an electronic configuration 2, 2. The compound formed when X combines with oxygen is most likely to be:
- a compound with a low melting point.
- a gas that dissolves in water to form an electrolyte.
- a good conductor in both solid and molten state.
- an ionic solid.
Answer
an ionic solid.
Reason — Electronic configuration 2, 2 means the atom has 2 electrons in its outer shell. Hence, it belongs to Group 2, it is a metal with valency 2 (it tends to lose 2 electrons to form X2+).
Oxygen is a non-metal with valency 2 (it tends to gain 2 electrons to form O2-).
So, when X combines with oxygen, the compound formed will be of the type X2+ O2-, i.e. metal + non-metal. This is ionic in nature and will exist as an ionic solid with high melting point, conducting electricity only in molten/solution state.
If an element has a low ionisation potential, it is most likely to be a:
- metal
- non-metal
- metalloid
- inert gas
Answer
Metal
Reason — The amount of energy required to remove a loosely bound electron from the outermost shell of an atom is known as ionisation potential. Metals have loosely held outer electrons and tend to lose electrons easily. Hence, they have low ionisation potential.

(a) Balance the chemical equation given below:
FeS2 + O2 ⟶ Fe2O3 + SO2
(b) Write balanced chemical equation for formation of ‘B’.
(c) Why is it necessary to convert ‘B’ to Oleum?
(d) Identify ‘C’.
(e) Write an equation for the reaction between Oleum and ‘C’.
Answer
(a) 4FeS2 + 11O2 ⟶ 2Fe2O3 + 8SO2
(b) Balanced chemical equation for formation of ‘B’
(c) Sulphuric acid is not obtained by directly reacting SO3 with water since sulphur trioxide does not dissolve in water satisfactorily and it gives a lot of heat and forms misty droplets of sulphuric acid. Hence, it is converted to oleum.
SO3 + H2SO4 (conc.) ⟶ H2S2O7 (oleum)
(d) C — Water (H2O)
(e) H2S2O7 + H2O ⟶ 2H2SO4
Ammonium hydroxide solution is added to the solution containing the ions mentioned in List X. List Y gives the details of the precipitate. Match the ions with their coloured precipitates.
| List X | List Y |
|---|---|
| (a) Zn2+ | 1. No visible reaction |
| (b) Fe2+ | 2. White precipitate insoluble in excess |
| (c) Pb2+ | 3. Gelatinous white precipitate soluble in excess |
| (d) Fe3+ | 4. Blue precipitate soluble in excess |
| (e) Ca2+ | 5. Dirty green precipitate insoluble in excess |
| 6. Reddish brown precipitate insoluble in excess |
Answer
| List X | List Y |
|---|---|
| (a) Zn2+ | 3. Gelatinous white precipitate soluble in excess |
| (b) Fe2+ | 5. Dirty green precipitate insoluble in excess |
| (c) Pb2+ | 2. White precipitate insoluble in excess |
| (d) Fe3+ | 6. Reddish brown precipitate insoluble in excess |
| (e) Ca2+ | 1. No visible reaction |
Complete the following sentences by choosing the correct answer from the brackets:
(a) The metal to be refined is kept at the ............... during the process of electro-refining. [cathode / anode]
(b) Dilute HCl and dilute H2SO4 can be distinguished by adding ............... solution. [NaNO3 / BaCl2]
(c) Ammonia gas is collected by ............... displacement of air. [upward / downward]
(d) The gas formed when copper carbonate is heated is ............... [O2 / CO2]
(e) Excess ammonia reacts with chlorine to form ............... [nitrogen / nitrogen trichloride]
Answer
(a) The metal to be refined is kept at the anode during the process of electro-refining.
(b) Dilute HCl and dilute H2SO4 can be distinguished by adding BaCl2 solution.
(c) Ammonia gas is collected by downward displacement of air.
(d) The gas formed when copper carbonate is heated is CO2.
(e) Excess ammonia reacts with chlorine to form nitrogen.
State the terms for the following:
(a) A substance which when dissolved in water forms hydronium ion as the only positive ion.
(b) A type of covalent bond in which electrons are shared equally between the combining atoms.
(c) The process by which a base metal is coated with another metal, either to protect the metal or to give it an attractive appearance.
(d) The type of reaction characteristic for alkanes.
(e) The substance which oxidises the other substance and itself gets reduced.
Answer
(a) Acid
(b) Non-polar covalent bond
(c) Electroplating
(d) Substitution reaction
(e) Oxidising agent
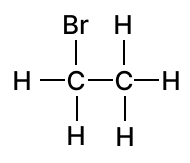
(a) Draw the structural diagram for the following organic compounds:
- bromoethane
- methanal
- but-2-yne
(b) Give IUPAC name for the following organic compounds:
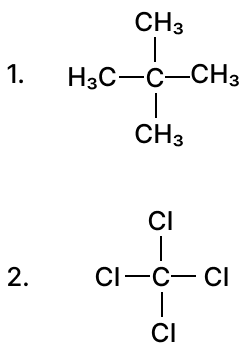
Answer
(a) Structural diagrams
1.
2. Structural formula of methanal is shown below:

3. Structural formula of But-2-yne is shown below:

(b) IUPAC names
- 2,2-dimethylpropane
- Tetrachloromethane
In the given equation
Zn + Pb2+ ⟶ Zn2+ + Pb
(a) ............... undergoes oxidation.
(b) ............... undergoes reduction.
Answer
(a) Zn undergoes oxidation.
Zn ⟶ Zn2+
(b) Pb2+ undergoes reduction.
Pb2+ ⟶ Pb
Justify the following statements:
(a) As one moves down a group, the reducing property of elements increases.
(b) Aluminium oxide cannot be reduced by carbon monoxide.
Answer
(a) Reducing property means the tendency to lose electrons, On moving down a group atomic size increases. Outer electrons are farther from the nucleus and less strongly attracted. Hence, as we move down the group elements lose electrons more readily, making them stronger reducing agents.
(b) Aluminium oxide is highly stable and aluminium has a greater affinity for oxygen than carbon, so CO is not strong enough to reduce Al2O3.
Arrange the following as per the instructions given in the brackets:
(a) Mg, S, Si, P [decreasing order of atomic size]
(b) Cl, I, Br, F [increasing electronegativity]
(c) K, Na, Rb, Li [decreasing metallic character]
Answer
(a) Mg > Si > P > S.
(b) I < Br < Cl < F
(c) Rb < K < Na < Li
Harsh performed the following experiments in the laboratory. State one significant observation made by Harsh when:
(a) he added concentrated sulphuric acid to blue vitriol.
(b) he passed ammonia gas over heated PbO.
(c) sodium hydroxide solution was added to CuSO4 solution by him.
Answer
(a) When Harsh added concentrated sulphuric acid to blue vitriol (CuSO4.5H2O), the blue colour crystals lose their blue colour and become white (anhydrous copper sulphate) this is because of loss of water molecules.
(b) When he passed ammonia gas over heated PbO, ammonia reduces heated yellow lead monoxide to greyish metallic lead.
2NH3 + 3PbO ⟶ 3Pb + 3H2O + N2 [g]
(c) On adding sodium hydroxide solution to CuSO4 solution, Harsh obtained a pale blue precipitate of Cu(OH)2 which was insoluble in excess of sodium hydroxide.
CuSO4 + 2NaOH ⟶ Cu(OH)2 + Na2SO4
Choose the letters L, M, N, O & P to match the description (a) to (c) given below:
[L – Ammonia, M – Nitrogen, N – Hydrogen sulphide, O – Hydrogen chloride gas, P – Nitrogen dioxide]
(a) When this gas comes in contact with ammonia dense white fumes are seen.
(b) The gas that turns moist lead acetate paper silvery black.
(c) The gas produced on heating lead nitrate.
Answer
(a) O — Hydrogen chloride gas
Reason — When HCl is brought near ammonia gas, dense white fumes of ammonium chloride are formed.
HCl + NH3 ⟶ NH4Cl
(b) N — Hydrogen sulphide
Reason — Hydrogen sulphide(H2S) gas turns the moist lead acetate paper black
(CH3COO)2Pb + H2S ⟶ PbS + CH3COOH
(c) P — Nitrogen dioxide
Reason — When lead nitrate is heated nitrogen dioxide gas is evolved.
2Pb(NO3)2 2PbO + O2 + 4NO2
Smith wrote the following statements incorrectly. Insert a word to correct the statements.
(a) Lead bromide conducts electricity.
(b) Copper reacts with nitric acid to form nitrogen dioxide gas.
(c) Bromoethane reacts with sodium hydroxide to produce ethanol and sodium bromide.
Answer
(a) Molten lead bromide conducts electricity.
Reason — Solid lead bromide is a non-conductor of electricity since it's ions are not free but held together by an electrostatic force of attraction. In molten state the ions become free and hence conduct electricity.
(b) Copper reacts with concentrated nitric acid to form nitrogen dioxide gas.
Reason — When copper reacts with dilute nitric acid it will form nitric oxide(NO) not nitrogen dioxide.
(c) Bromoethane reacts with aqueous sodium hydroxide to produce ethanol and sodium bromide.
Reason — Alcohol is prepared by the hydrolysis of alkyl halides (haloalkanes) on reaction with aqueous NaOH. Hydrolysis of bromoethane occurs with aqueous NaOH, not anhydrous/alcoholic NaOH.
Match the Column A (showing the properties of H2SO4) with Column B (showing the reaction of H2SO4)
| Column A Properties of H2SO4 | Column B Reaction of H2SO4 |
|---|---|
| (a) Acidic property | 1. C12H22O11 + nH2SO4 ⟶ 12C + 11H2O + nH2SO4 |
| (b) Dehydrating property | 2. S + 2H2SO4 ⟶ 3SO2 + 2H2O |
| (c) Non-volatile acid | 3. CaO + H2SO4 ⟶ CaSO4 + H2O |
| (d) Oxidizing agent | 4. NaCl + H2SO4 ⟶ NaHSO4 + HCl |
Answer
| Column A Properties of H2SO4 | Column B Reaction of H2SO4 |
|---|---|
| (a) Acidic property | 3. CaO + H2SO4 ⟶ CaSO4 + H2O |
| (b) Dehydrating property | 1. C12H22O11 + nH2SO4 ⟶ 12C + 11H2O + nH2SO4 |
| (c) Non-volatile acid | 4. NaCl + H2SO4 ⟶ NaHSO4 + HCl |
| (d) Oxidizing agent | 2. S + H2SO4 ⟶ 3SO2 + 2H2O |
Calcium oxide is a drying agent which removes water vapour. A student wanted to collect a dry sample of the hydrogen chloride gas produced. The student set up the apparatus as shown below but was unsuccessful in collecting any gas.
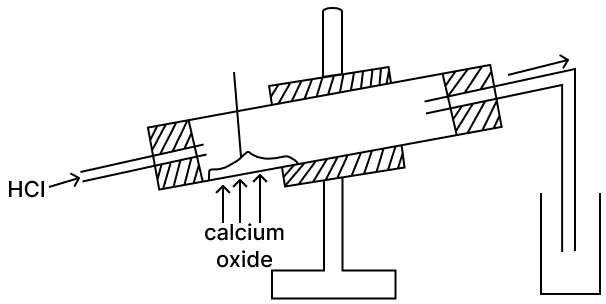
(a) What mistake did the student make?
(b) What change should be made by the student in order to collect the dry HCl gas?
Answer
(a) The student was unsuccessful in collecting any gas because calcium oxide (quick lime) react with hydrogen chloride.
CaO + 2HCl ⟶ CaCl2 + H2O
(b) The student can use conc. sulphuric acid as a drying agent. Conc. H2SO4 absorbs water vapour but does not react with HCl gas.
Select the correct answer from the options given in the brackets:
(a) The ion which is discharged at the cathode during the electrolysis of CuSO4 solution using copper electrodes. [Cu2+, OH-, SO42-, H+]
(b) During electroplating of an article with Ag using sodium argentocyanide as an electrolyte, the anode is made of. [Cu, Ag, Pt, Na]
Answer
(a) Cu2+
Reason — During the electrolysis of CuSO4 solution using copper electrodes. Both Cu2+ and H+ ions migrate to the cathode, Cu2+ ions discharged as neutral copper atoms.
(b) Ag
Reason — During electroplating of an article with Ag using sodium argentocyanide as an electrolyte, the anode is the plate of pure clean silver.
Ethane C2H6 burns in oxygen to produce carbon dioxide and water as shown in the equation given below:
2C2H6 + 7O2 ⟶ 4CO2 + 6H2O
Calculate the composition of the resulting gaseous mixture at room temperature when 60 c.c. of ethane burns in 250 c.c. of oxygen.
Answer
[By Gay Lussac's law]
2 Vol. of C2H6 requires 7 Vol. of oxygen
∴ 60 cc C2H6 will require x 60
= 210 cc of Oxygen
Hence, unused oxygen = 250 - 210 = 40 cc
Similarly,
2 Vol. of C2H6 produces 4 Vol. of carbon dioxide
∴ 60 cc C2H6 produces x 60
= 120 cc of Carbon dioxide
Hence, carbon dioxide produced = 120 cc.
The gaseous mixture at room temperature (assuming water condenses) contains 120 cc CO2 and 40 cc O2.
Match the uses of alloys in List 1 with the appropriate answer from List 2.
| List 1 | List 2 |
|---|---|
| (a) Used in making decorative articles. | 1. Stainless steel |
| (b) An alloy used in making aircraft and light tools. | 2. Brass |
| (c) Used in making surgical Instruments. | 3. Duralumin |
Answer
| List 1 | List 2 |
|---|---|
| (a) Used in making decorative articles. | 2. Brass |
| (b) An alloy used in making aircraft and light tools. | 3. Duralumin |
| (c) Used in making surgical Instruments. | 1. Stainless steel |
You are provided with some compounds in the box.
[SO2, PbO, CO, K2SO4, H2SO4, CH3COOH, NaHSO4, KCl]
Choose the compound from the above box that fits the descriptions from (a) to (c).
(a) An acid present in vinegar.
(b) An oxide which dissolves in water forming an acid.
(c) A salt formed by the incomplete neutralization of an acid by a base.
Answer
(a) CH3COOH
(b) SO2
(c) NaHSO4
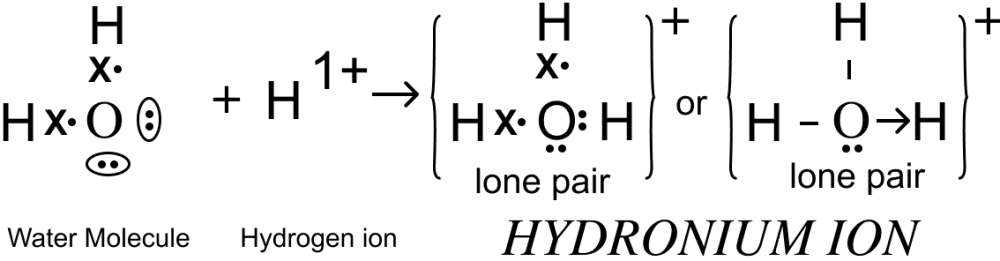
Draw the dot and cross structure of the following:
(a) Hydronium ion
(b) Oxygen molecule
(c) Calcium oxide
[Atomic number: H = 1, O = 8, Ca = 20]
Answer
(a) Electron dot diagram of hydronium ion is shown below:
(b) Electron dot diagram of oxygen molecule is shown below:

(c) Electron dot diagram of Calcium oxide is shown below:

Given below is the diagram for the laboratory preparation of Nitric Acid.

(a) Name the reactant labelled Y.
(b) Write a balanced equation for the reaction between Y and KNO3.
(c) The complete apparatus is made up of glass. Why?
(d) State why concentrated HNO3 appears slightly yellowish in colour when left standing in a glass bottle for a long time.
Answer
(a) Conc. sulphuric acid
(b)
(c) The complete apparatus is made up of glass because nitric acid vapours attack rubber and cork.
(d) Pure acid is colourless but the acid obtained in the laboratory is slightly yellow. The yellow colour is due to dissolution of reddish brown coloured nitrogen dioxide gas in the acid. This gas is produced due to the thermal decomposition of a portion of nitric acid.
4HNO3 ⟶ 2H2O + 4NO2 + O2
Answer the following questions related to the electrolytic reduction of pure Alumina by Hall Heroult’s process.
(a) The reaction occurring at the anode.
(b) The reaction occurring at the cathode.
Answer
(a) At anode : 6O2- - 12e- ⟶ 3O2
(b) At cathode : 4Al3+ + 12e- ⟶ 4Al
Give the chemical formula of the following ores:
(a) Cryolite
(b) Haemetite
Answer
(a) Cryolite — Na3AlF6
(b) Haemetite — Fe2O3
Write the balanced equations for the following reactions:
(a) Potassium bicarbonate reacts with dilute HCl.
(b) Laboratory preparation of ethane using soda lime.
(c) Warm water is added to Aluminium nitride.
Answer
(a) KHCO3 + HCl ⟶ KCl + H2O + CO2 ↑
(b)
(c) AlN + 3H2O ⟶ Al(OH)3 + NH3 ↑
Nitrogen and hydrogen combine in the presence of a catalyst to give ammonia gas. With reference to the above reaction:
(a) Name the catalyst used.
(b) At what temperature does the above reaction occur?
(c) What optimum pressure should be maintained during the reaction?
Answer
(a) Catalyst — Finely divided iron [Fe]
(b) Temperature — 450-500°C [Optimum temperature]
(c) Pressure — 200 to 900 atmospheres [Optimum pressure]
1 mole of CO2 occupies 24 dm3 at room temperature and pressure. Calculate the following:
(a) The mass of 6 litres of CO2.
(b) The volume occupied by 60 g of CO2.
[Atomic weight: C=12, O=16]
Answer
Given:
1 mole of CO2 occupies 24 dm3 (24 L) at room temperature and pressure
Gram molecular mass of CO2 = 12 + 2 x 16 = 44 g
(a) 24 litres of CO2 weighs = 44 g (at room temperature & pressure)
∴ Mass of 6 litres of CO2 = x 6 = 11 g (at room temperature & pressure)
Hence, mass of 6 litres of CO2 at room temperature & pressure is 11 g.
(b) Volume occupied by 44 g of CO2 = 24 litres (at room temperature & pressure)
∴ Volume occupied by 60 g of CO2 = x 60 ≈ 32.72 litres (at room temperature & pressure)
Hence, volume occupied by 60 g of CO2 ≈ 32.72 litres (at room temperature & pressure)
Define:
(a) Electrolyte
(b) Catenation
Answer
(a) Electrolyte is a compound which either in aqueous solution or in molten state allow electric current to pass through it.
(b) The property of self linking of atoms of an element through covalent bonds in order to form straight chains, branched chains and cyclic chains of different sizes is known as catenation.
Name the most appropriate method of preparation of the following salts:
(a) Copper carbonate
(b) Sodium sulphate
(c) Ferric chloride
Answer
(a) Copper carbonate — Double Decomposition (Precipitation)
(b) Sodium sulphate — Neutralisation
(c) Ferric chloride — Direct combination
Complete and balance the following equation:
(a) C2H5Br + KOH (alcoholic) ⟶
(b) C2H5OH
(c) CH4 + Cl2 ⟶
Answer
(a) C2H5Br + KOH (alcoholic) ⟶ C2H4 + KBr + H2O
(b) 2C2H5OH C2H5 — O — C2H5 + H2O
(c) CH4 + Cl2 ⟶ CH3Cl + HCl