Which of the following will dissociate in aqueous solution, to give a positive ion other than hydronium ion, and a negative ion other than hydroxyl ion?
- KOH
- dil. HCl
- NaCl
- CH3COOH
Answer
NaCl
Reason — NaCl dissociates in water into positive ion Na+ other than hydronium ion, and a negative ion Cl- other than hydroxyl ion. Whereas, KOH gives hydroxyl ion and HCl and CH3COOH gives hydronium ion in water.
A compound P is heated in a test tube with sodium hydroxide solution. A red litmus paper held at the mouth of the test tube turns blue. Which of the following could compound P be?
- Zinc sulphate
- Copper sulphate
- Ferrous sulphate
- Ammonium sulphate
Answer
Ammonium sulphate
Reason — Ammonium sulphate reacts with sodium hydroxide to produce sodium sulphate, ammonia gas, and water. The red litmus turns blue due to the basic ammonia gas.
Assertion (A): Aqueous solution of potassium chloride can conduct electricity.
Reason (B): Conduction of electric current is due to the presence of free ions.
- (A) is true and (R) is false.
- (A) is false and (R) is true.
- Both (A) and (R) are true and (R) is the correct explanation of (A).
- Both (A) and (R) are true, but (R) is not the correct explanation of (A).
Answer
Both (A) and (R) are true and (R) is the correct explanation of (A).
Reason — KCl is an ionic compound. In water, it dissociates completely into K+ and Cl- ions. These free moving ions carry electric current. Hence, the assertion (A) is true. The principle behind electrolytic conduction in solutions is the presence of free ions. Aqueous solution of KCl conduct electricity due to the presence of free ions. Hence, the reason (R) is true and it correctly explains assertion (A).
Identify the ion that contain one lone pair of electrons.
- OH-1
- H3O+
- NH4+
- H+
Answer
H3O+
Reason — The pair of electrons not shared by any other atom are called lone pair of electrons. H3O+ has only one lone pair of electrons. Whereas, OH1- contains three lone pair of electrons and NH4+ and H+ contains no lone pair of electrons.
Four reactions are shown below in the diagram:

Which reactions produce water?
- 1 and 2
- 1 and 3
- 3 and 4
- 2 and 3
Answer
3 and 4
Reason — Reaction 3 shows burning of ethanol, which is an example of combustion and combustion produces water and carbon dioxide. Reaction 4 has dilute sulphuric acid and magnesium carbonate, a reaction of acid and carbonate produces salt, water and carbon dioxide. Hence, reaction 3 and reaction 4 produces water.
When compound X reacts with dilute sulphuric acid, it releases a gas that turns acidified potassium dichromate solution from orange to green. Which of the following could be compound X?
- Lead nitrate
- Copper carbonate
- Sodium chloride
- Potassium sulphite
Answer
Potassium sulphite
Reason — Potassium sulphite reacts with dilute sulphuric acid, to release sulphur dioxide. SO2 is a reducing gas, that turns acidified potassium dichromate solution from orange to green.
K2SO3 + H2SO4 ⟶ SO2 ↑ + H2O + K2SO4
The volume occupied by 2 moles of a gas at STP is:
- 22.4L
- 2.24L
- 44.8L
- 4.48L
Answer
44.8L
Reason — One mole of gas occupies 22.4 L of volume at STP
∴ Volume occupied by 2 moles of gas = 2 x 22.4 L = 44.8 L
Identify from the following metal oxide which can react with an acid as well as an alkali.
- Silver oxide
- Calcium oxide
- Copper(II) oxide
- Aluminium oxide
Answer
Aluminium oxide
Reason — Amphoteric oxides like aluminium oxide react with acids as well as alkalis giving salt and water.
The structures of four hydrocarbons are shown below:

How many isomers of butene are shown in the above structures?
- 1
- 2
- 3
- 4
Answer
2
Reason — Among the four hydrocarbon structure show, structure 3 and 4 are isomers of butene. Structure 3 is But-1-ene and structure 4 is 2-methyl propene.
Which element amongst the following has the largest atomic radius?
- Al
- S
- Mg
- Na
Answer
Na
Reason — Aluminium (Al), Sulphur (S), Magnesium (Mg) and Sodium (Na) belong to third period. Atomic size decreases on moving across the period as new electrons are added to the same shell. Sodium is the first member of period 3, hence sodium has the largest atomic radius of the given elements.
For which pH change is there the maximum increase in acidity?
| Initial pH | Final pH | |
|---|---|---|
| 1. | 1 | 3 |
| 2. | 2 | 6 |
| 3. | 3 | 1 |
| 4. | 6 | 2 |
Answer
| 4. | 6 | 2 |
Reason — According to the pH scale, lesser the pH more is the acidity. When pH decreases from 6 to 2, the amount of H+ increases 10,000 times.
pH= −log10 [H+ ] ⟶ [H+ ] = 10-pH
Initial [H+] = 10-6 = 0.000001 mol/L
Final [H+] = 10-2 = 0.01 mol/L
Change: = 10,000 times more acidic
The equation below shows the reaction between element ‘X’ and dilute sulphuric acid.
X(s) + H2SO4 (aq.) ⟶ XSO4 (aq.) + H2 (g)
Which particles are responsible for conducting electricity in dilute sulphuric acid and compound XSO4?
- Electrons
- Only positive ions
- Only negative ions
- Both positive and negative ions
Answer
Both positive and negative ions
Reason — Sulphuric acid will dissociate into H+ and SO42-. Also, in aqueous solution, the compound XSO4 would dissociate into X+ and SO42-. In both cases, both positive and negative ions are responsible for conducting electricity.
Methanol and ethanol belong to the same homologous series. What does this statement mean?
- Their molecules contain atoms only of carbon and hydrogen.
- Their molecules have the same number of carbon atoms.
- They have the same functional group.
- They have the same relative molecular mass.
Answer
They have the same functional group.
Reason — Methanol and ethanol belong to the same homologous series. They share the general formula and same functional group. Ethanol differs methanol by CH2 group. Relative molecular mass and number of carbon atoms in successive members of homologous series increases.
The ratio between the volumes occupied by 22 grams of carbon dioxide and 10 grams of hydrogen gas is:
- 2.2 :1
- 1: 2.2
- 1:10
- 10:1
Answer
1:10
Reason — Molar mass of CO2 = 12 + 2 x 16 = 44 g/mole
∴ Moles of 22g CO2 = = 0.5
Molar mass of H2 = 2 x 1 = 2 g/mole
∴ Moles of 10g H2 = = 5
Under the same conditions of temperature and pressure, the volume of a gas is directly proportional to the number of moles. Therefore, the volume ratio will be the same as the mole ratio.
Volume ratio = = = 1 : 10
In the process of Electrorefining of Copper shown in the diagram below, which of the following statements is correct?

- The anode is made of pure Copper.
- The cathode is made of impure Copper.
- Copper is deposited at the anode.
- Copper ions from the anode move to the cathode and get deposited as pure Copper.
Answer
Copper ions from the anode move to the cathode and get deposited as pure Copper.
Reason — At the anode, impure copper dissolves into the solution as Cu2+ ions. These Cu2+ ions migrate through the solution to the cathode, where they gain electrons and are deposited as pure copper.
Electroplating steel objects with silver involves a three-step process.

step 1 — A coating of copper is applied to the object.
step 2 — A coating of nickel is applied to the object.
step 3 — The coating of silver is applied to the object.
(a) A diagram of the apparatus used for step 1 is shown.
- The chemical process taking place on the surface of the object is
Cu2+ (aq) + 2e- ⟶ Cu(s)
What is the observation seen on the surface of the object? - Explain why the concentration of copper ions in the electrolyte remains constant throughout step1.
(b) Give two changes which would be needed in order to coat nickel onto the object in step 2.
(c) Write down the reaction taking place at the positive electrode during step 3.
Answer
(a)
- The object will develop a coating of copper. Visually, this results in the object taking on a reddish-brown appearance due to the deposition of metallic copper. Also there is an increase in mass of the steel object.
- As copper ions are reduced and deposited onto the object, an equal amount of copper metal is oxidised from the anode to maintain the concentration of Cu2+ in the electrolyte solution.
(b) Two changes which would be needed in order to coat nickel onto the object in step 2 are:
- Replace the copper sulphate solution with a nickel-containing electrolyte solution, such as nickel sulphate.
- Replace the copper anode with a nickel anode.
(c) Ag (s) ⟶ Ag+ (aq) + e-
Identify the following:
(a) A bond formed between two atoms by sharing of a pair of electrons, with both electrons being provided by the same atom.
(b) A salt formed by the complete neutralisation of an acid by a base.
(c) A reaction in which the hydrogen of an alkane is replaced by a halogen.
(d) The energy required to remove an electron from a neutral gaseous atom.
(e) A homogenous mixture of two or more metals or a metal and a non- metal in a definite proportion in their molten state.
Answer
(a) Coordinate bond
(b) Normal salt
(c) Substitution/ Halogenation
(d) Ionisation potential
(e) Alloy
Complete the following by choosing the correct answers from the bracket:
(a) When dilute sulphuric acid reacts with zinc granules, the gas evolved is ............... (hydrogen / carbon dioxide), which can be tested using a burning splint.
(b) A solution of copper(II) sulphate in sodium hydroxide solution forms a ............... (pale blue / green) precipitate.
(c) In methane, each hydrogen atom share(s) ............... (one / two) electron(s) with the central carbon atom to complete its valence shell.
(d) The electron affinity of element X is greater than that of element Y. The oxidising power of X is likely to be ............... (more / less) than that of element Y.
(e) The naturally occurring compound of a metal from which the metal can be extracted is called its ............... (ore / mineral).
Answer
(a) When dilute sulphuric acid reacts with zinc granules, the gas evolved is hydrogen, which can be tested using a burning splint.
(b) A solution of copper(II) sulphate in sodium hydroxide solution forms a pale blue precipitate.
(c) In methane, each hydrogen atom share one electron with the central carbon atom to complete its valence shell.
(d) The electron affinity of element X is greater than that of element Y.The oxidising power of X is likely to be more than that of element Y.
(e) The naturally occurring compound of a metal from which the metal can be extracted is called its ore.
Match Column A with Column B.
| Column A | Column B |
|---|---|
| (a) Aluminium | 1. Covalent compound |
| (b) Sulphuric acid | 2. Carbonate ore |
| (c) Calcination | 3. Hall Heroults process |
| (d) Calcium chloride | 4. Contact process |
| (e) Carbon tetrachloride | 5. Electrovalent compound |
Answer
| Column A | Column B |
|---|---|
| (a) Aluminium | 3. Hall Heroults process |
| (b) Sulphuric acid | 4. Contact process |
| (c) Calcination | 2. Carbonate ore |
| (d) Calcium chloride | 5. Electrovalent compound |
| (e) Carbon tetrachloride | 1. Covalent compound |
(a) Give the IUPAC name of the following organic compounds:
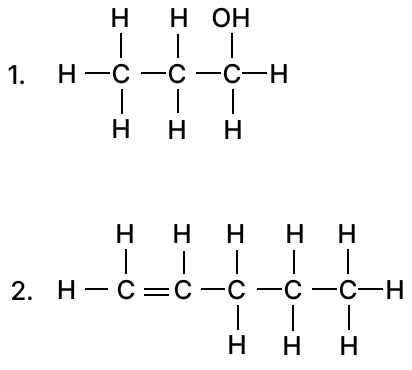
(b) Draw the structural diagram for the following compounds:
- but-2-yne
- 1, 1, 1, trichloro methane
- pentan-2-ol
Answer
(a) IUPAC name of the following organic compounds are :
- propanol
- pentene
(b) Structural formula
1. But-2-yne

2. 1, 1, 1, trichloro methane

3. Pentan-2-ol

Give one significant observation when:
(a) Excess of chlorine gas reacts with ammonia.
(b) Zinc nitrate is strongly heated in a test tube.
Answer
(a) Colourless ammonia gas reacts with greenish yellow excess chlorine giving a yellow explosive liquid (Nitrogen trichloride).
NH3 + 3Cl2 [excess] ⟶ 3HCl + NCl3
(b) Reddish brown nitrogen dioxide gas is evolved on heating zinc nitrate crystals.
Give reasons:
(a) When ammonia gas is passed over black copper oxide in a combustion tube a reddish-brown substance is left behind.
(b) Quick lime is not used to dry hydrogen chloride gas.
Answer
(a) When ammonia gas is passed over black copper oxide in a combustion tube a reddish-brown substance is left behind because ammonia gas reduces black copper [II] oxide to brown Copper.
2NH3 + 3CuO ⟶ 3Cu + 3H2O + N2 ↑
(b) Quick lime is not used to dry hydrogen chloride gas because quick lime reacts with hydrogen chloride gas
The electron affinity of an element X is greater than that of element Y.
(a) How is the oxidising power of X likely to compare with that of Y?
(b) How is the electronegativity of X likely to compare with that of Y?
(c) State whether X is likely to be placed to the left or to the right of Y in the periodic table?
Answer
(a) Oxidising power of X > Y. Elements with high electron affinity accept electrons more easily hence have greater oxidising power.
(b) Electronegativity of X > Y. Element with high electron affinity has high electronegativity.
(c) X is to the right side of Y as electron affinity increases from left to right in a period.
Write balanced chemical equations for the following reactions:
(a) Ammonium chloride reacts with calcium hydroxide.
(b) Nitric acid reacts with zinc carbonate.
(c) Concentrated sulphuric acid is added to hydrated copper sulphate.
Answer
(a) 2NH4Cl + Ca(OH)2 ⟶ CaCl2 + 2H2O + 2NH3
(b) 2HNO3 + ZnCO3 ⟶ Zn(NO3)2 + H2O + CO2
(c) H2SO4 + CuSO4. 5H2O ⟶ CuSO4 + 5H2O + H2SO4
Name the main metal present in the following alloys:
(a) Duralumin
(b) Brass
Answer
(a) Aluminium
(b) Copper
Write balanced chemical equations for the following:
(a) Laboratory preparation of hydrochloric acid from a less volatile acid.
(b) Bromine gas is passed over ethene in the presence of carbon tetrachloride.
Answer
(a) NaCl + conc. H2SO4 ⟶ NaHSO4 + HCl
(b) C2H4 + Br2 ⟶ C2H4Br2
Abhishek was given a salt ‘X’ for analysis which was white in colour. On strong heating it produced a yellow residue, a colourless gas, and also a reddish-brown gas. The solution of the salt ‘X’ when tested with excess of ammonium hydroxide produced a chalky white insoluble precipitate.
(a) Name the coloured gas evolved when Abhishek heated the salt strongly.
(b) Which cation was present in the sample given to Abhishek?
(c) Identify the salt given to Abhishek for analysis.
Answer
(a) Nitrogen dioxide
Reason — The reddish-brown colour indicates that the gas is Nitrogen dioxide.
(b) Lead ion (Pb2+)
Reason — The chalky white insoluble precipitate with excess of ammonium hydroxide indicates that the cation present is Pb2+.
(c) Lead nitrate[Pb(NO3)2]
Reason — The given salt is a nitrate salt as nitrogen dioxide (NO2), is a common product obtained from heating nitrates. As the cation is lead, hence the salt is Lead nitrate.
In a round bottom flask, a mixture of ethanol, acetic acid and concentrated sulphuric acid was heated:
(a) Name the type of reaction occurring in the above set up.
(b) What is the role of sulphuric acid in this reaction?
(c) State one observation that takes place during the reaction.
Answer
(a) Esterification reaction
(b) Dehydrating agent
(c) Fruity smell is observed due to the formation of ester by the reaction of ethanol and acetic acid.
Identify the reactant and write the balanced equation for the following:
Nitric acid reacts with compound Q to give a salt Calcium nitrate, water and carbon dioxide.
Answer
Q is Calcium carbonate or Calcium bicarbonate
CaCO3 + 2HNO3 ⟶ Ca(NO3)2 + H2O + CO2
or
Ca(HCO3)2 + 2HNO3 ⟶ Ca(NO3)2 + 2H2O + 2CO2
What will be the mass of carbon dioxide that will contain the same number of molecules as present in 3.2g of oxygen gas? [At. Wt: O=16, C=12]
Answer
Molecular mass of O2 = 2 × 16 = 32 g/mol
Given mass = 3.2 g of Oxygen
Moles of O2 = = = 0.1 mol
∴ 0.1 mol of O2 contain = 0.1 x 6.023 × 1023 of molecules.
We need the same number of molecules of CO2
∴ Moles of CO2 will also be 0.1 mol
Molar mass of CO2 = 12 + 2(16) = 12 + 32 = 44 g/mol
Mass = moles x molar mass
Mass of CO2 = 0.1 x 44 = 4.4 g.
∴ Mass of CO2 = 4.4 g
State the property exhibited by sulphuric acid in each of the following reactions:
(a) Sulphur with concentrated sulphuric acid.
(b) Conversion of ferrous sulphide to hydrogen sulphide gas using sulphuric acid.
(c) Ethanol with concentrated sulphuric acid.
Answer
(a) Oxidising property
(b) Acidic property
(c) Dehydrating property
Give balanced equations for the following:
(a) Laboratory preparation of ethyne from calcium carbide.
(b) Conversion of acetic acid to ethyl acetate.
(c) Laboratory preparation of nitric acid.
Answer
(a) CaC2 + 2H2O ⟶ Ca(OH)2 + C2H2
(b) CH3COOH + C2H5OH ⟶ CH3COOC2H5 + H2O
(C) NaNO3 + conc. H2SO4 ⟶ NaHSO4 + 2HNO3
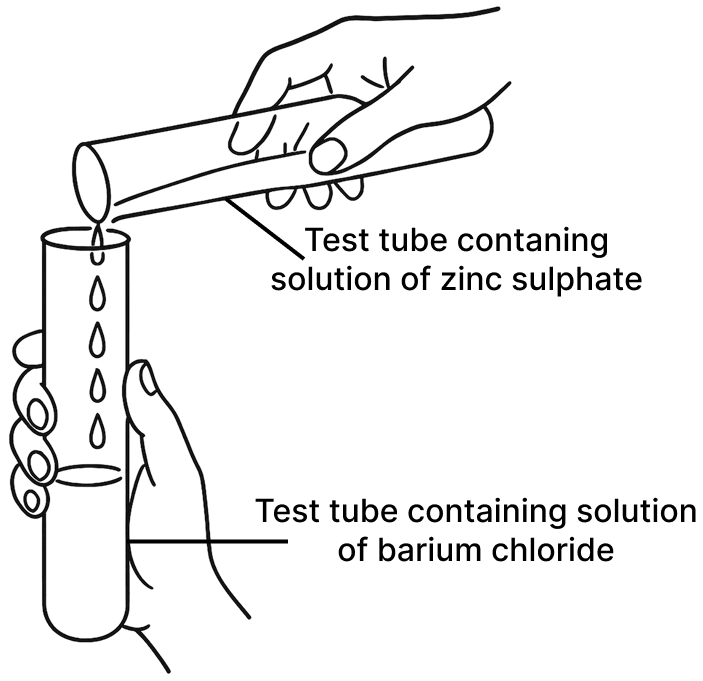
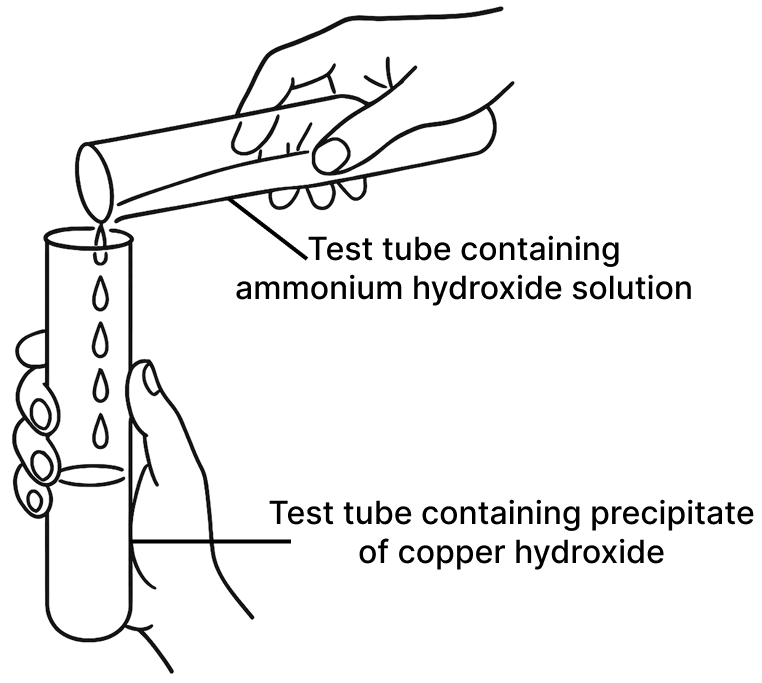
A student was asked to perform two experiments in the laboratory based on the instructions given: Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
(a) Experiment 1
(b) Experiment 2
Answer
(a) White precipitate of barium sulphate is formed.
Reason — When a solution of zinc sulphate is added to a solution of barium chloride, a double displacement reaction occurs. This reaction involves the exchange of ions between the two compounds and a white precipitate of barium sulphate is formed.
ZnSO4 (aq) + BaCl2 (aq) ⟶ BaSO4 (s) + ZnCl2 (aq).
(b) Blue precipitate dissolves to form an inky blue/deep blue solution.
Reason — The light blue precipitate of copper(II) hydroxide (Cu(OH)2) dissolves in excess of ammonium hydroxide due to the formation of a deep blue complex, tetraamminecopper(II) ([Cu(NH3)4]2+), making it soluble.
You are provided with the list of chemicals mentioned below in the box:
| Sodium hydroxide solution, copper carbonate, zinc, hydrochloric acid, copper, dilute sulphuric acid |
|---|
Using suitable chemicals from the list given, write balanced chemical equation for the preparation of the salts mentioned below:
(a) copper sulphate
(b) sodium zincate
Answer
(a) CuCO3 + H2SO4 ⟶ CuSO4 + H2O + CO2
(b) Zn + 2NaOH ⟶ Na2ZnO2 + H2
Solid ammonium dichromate decomposes as under:
(NH4)2Cr2O7 ⟶ N2 + Cr2O3 + 4H2O
If 126 g of ammonium dichromate decomposes, calculate:
(a) the number of moles of ammonium dichromate that undergoes decomposition.
(b) the mass of chromic oxide formed at the same time.
(c) the volume of nitrogen gas evolved at STP.
[At. Wt: N=14, Cr =52, O=16, H=1]
Answer
(a) 252 g of (NH4)2Cr2O7 = 1 mole
∴ 126 g of (NH4)2Cr2O7 = x 126 = 0.5 moles
Hence, no. of moles = 0.5 moles
(b) 252 g of (NH4)2Cr2O7 gives 152 g of Cr2O7
126 g of (NH4)2Cr2O7 will give = 76 g of Cr2O7
Hence, mass in gms of Cr2O7 formed = 76 g.
(c) 252 g of (NH4)2Cr2O7 produces 22.4 lit of N2
126 g of (NH4)2Cr2O7 will produce = 11.2 lit of N2
Hence, volume of N₂ evolved at s.t.p = 11.2 lit.
Identify the reactants P, Q and R in the following reactions:
(a) Copper oxide + P ⟶ Copper + water
(b) Iron pyrite + Q ⟶ Iron oxide + Sulphur dioxide
(c) Sodium chloride + R ⟶ Sodium nitrate + Silver chloride
Answer
(a) Hydrogen
(b) Oxygen
(c) Silver Nitrate
Give reasons for the following:
(a) Nitric acid does not normally liberate hydrogen gas when it reacts with active metals.
(b) Silver-plated cutlery is not considered as pure silver.
Answer
(a) Nitric Acid is a very strong oxidising agent and hence oxidises hydrogen to water. So, it is not used for obtaining hydrogen from metals.
(b) Silver plated cutlery is not considered as pure silver because it is not made fully of silver only its top coating is of silver.
The following questions relate to the extraction of Aluminium by electrolysis.
(a) Name the other compound which contains aluminium added to alumina.
(b) Give a balanced equation for the reaction that takes place at the cathode.
Answer
(a) Cryolite
(b) 2Al3+ + 6e- ⟶ 2Al
Give balanced equations for each of the following:
(a) Action of warm water on aluminium nitride.
(b) Oxidation of carbon with conc. nitric acid.
(c) Laboratory preparation of ethanol by using chloroethane and aqueous sodium hydroxide.
Answer
(a) AlN + 3H2O ⟶ Al(OH)3 + NH3
(b) C + 4HNO3 ⟶ CO2 + 2H2O + 4NO2
(c) C2H5Cl + aq. NaOH ⟶ C2H5OH + NaCl
Rohit has solution X, Y and Z that has pH 2, 7 and 13 respectively. Which solution:
(a) will liberate sulphur dioxide gas when heated with sodium sulphite?
(b) will liberate ammonia gas when reacted with ammonium chloride?
(c) will not have any effect on litmus paper?
Answer
(a) X
Reason — Sodium sulphite can react with acids to liberate sulphur dioxide gas. So, the solution X with pH 2 (acidic) will react with sodium sulphite to liberate sulphur dioxide gas. Na2SO3 + 2H- ⟶ SO 2+ H2O + 2Na+
(b) Z
Reason — Ammonium chloride is a salt that, in the presence of a base, will release ammonia gas. Therefore, a basic solution (with a high pH) will react with ammonium chloride to liberate ammonia gas. Thus, the solution Z with pH 13 (basic) will liberate ammonia gas when reacted with ammonium chloride. NH4Cl + OH- ⟶ NH3 + H3O + Cl-
(c) Y
Reason — The solution Y with pH 7 will not have any effect on litmus paper as it is neither acidic nor basic.
State giving reasons if:
(a) zinc metal and aluminium metal can be distinguished by heating the metal powders separately in two different test tubes with concentrated sodium hydroxide solution.
(b) calcium nitrate and lead nitrate can be distinguished by adding ammonium hydroxide solution to the salt solution.
Answer
(a) Zinc and aluminium cannot be distinguished by heating the metal powder with concentrated sodium hydroxide solution as they both react with conc. alkalis to form soluble sodium salts and hydrogen gas.
Zinc reacts to form sodium zincate
Zn + 2NaOH ⟶ H2 + Na2ZnO2 [sodium zincate]
Aluminium reacts to form sodium aluminate
2Al + 2NaOH + 2H2O ⟶ 3H2 + 2NaAlO2 [sodium aluminate]
(b) Yes, calcium nitrate and lead nitrate can be distinguished using ammonium hydroxide solution. Ammonium hydroxide on reaction with lead nitrate gives chalky white precipitate of Pb(OH)2. No precipitation occurs on adding Ammonium hydroxide to calcium nitrate even when it is added in excess.
Pb(NO3)2 + 2NH4OH ⟶ Pb(OH)2 + 2NH4NO3
Draw the electron dot diagram of ammonium ion. [Atomic No.: N = 7, H = 1]
Answer
Electron dot diagram of ammonium ion is shown below:

Write balanced chemical equation for the following conversions (A to C):

Answer
A = Zn + H2SO4 ⟶ ZnSO4 + H2
B = Zn + S ⟶ ZnS
C = ZnSO4 + 2NaOH ⟶ Zn(OH)2 + Na2SO4
L, M and N are three elements with atomic numbers 13, 7 and 10 respectively. Answer the following questions using only the alphabets given. Do not identify the elements.
Which element:
(a) can combine with hydrogen to form a gas which produces dense white fumes with concentrated HCl?
(b) has zero electron affinity?
(c) can form an ionic compound with oxygen?
Answer
(a) M
Reason — Atomic number of element M is 7 so its electronic configuration is 2,5.Hence, it is a non-metal with 5 valence electrons. It combines with hydrogen to form a covalent hydride, MH3, which is a basic gas. MH3 reacts with concentrated HCl to give dense white fumes of ammonium salt.
(b) N
Reason — Atomic number of element N is 10 so its electronic configuration is 2, 8. It has electron affinity zero as it's octet is complete and it is a stable element.
(c) L
Reason — Atomic number of element L is 7 so its electronic configuration is 2, 8, 3. Hence, it is a metal with 3 valence electrons. Metals tend to lose electrons, and non-metals like oxygen tend to gain electrons, so L can transfer its 3 valence electrons to oxygen. This forms L3+ ions and O2- ions, giving an ionic compound with oxygen, generally of the form L2O3. So, L is the element that can form an ionic compound with oxygen.