Chemistry
Assertion (A): Ammonia is dried by passing through a drying tower containing CaO.
Reason (R): Ammonia being basic in nature reacts with other drying agents.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Ammonia
7 Likes
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— Ammonia is dried by passing it through a drying tower containing lumps of quicklime (CaO). Hence, the assertion (A) is true.
Other drying agents like conc. sulphuric acid, phosphorous pentoxide and anhydrous calcium chloride are not used, as ammonia is basic in nature, reacts with other drying agents. Hence, the reason (R) is true and it is the correct explanation of assertion (A).
Answered By
5 Likes
Related Questions
In the following set up for burning ammonia in oxygen, ammonia tube is fitted higher and oxygen tube is kept lower because ?
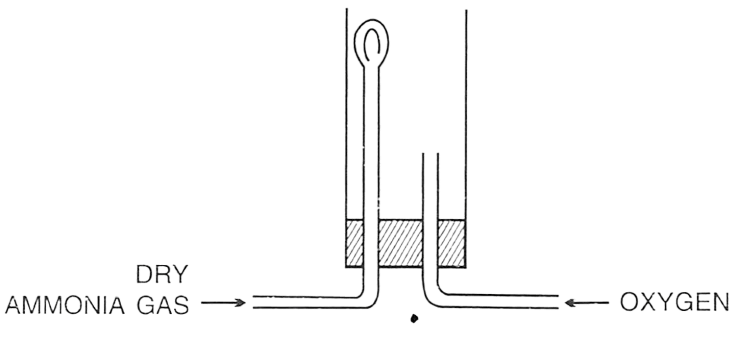
P — Oxygen is lighter than ammonia.
Q — Ammonia is lighter than oxygen.
R — Ammonia and oxygen mixture is explosive and by keeping them in this position, limited oxygen will react with ammonia.
Which of the following is true ?
- Only P
- Only Q
- Only R
- Both P and Q
Assertion (A): Ammonia does not conduct electricity in gaseous or liquid state.
Reason (R): Ions in ammonia gas or liquid ammonia are not free to move.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): Ammonia and its compounds do not occur in minerals.
Reason (R): Ammonia is not soluble in water.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): In the preparation of ammonia, hydrogen is obtained by Bosch process.
Reason (R): Hydrogen and nitrogen are used in the ratio 1:3.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.