Chemistry
Assertion (A): The atomic mass of an element is mainly the mass of its nucleus.
Reason (R): The nucleus of an element contains neutrons and protons and the electrons have a negligible mass.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Atomic Structure
4 Likes
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Mass number of an element is the total number of protons and neutrons present in its nucleus. Hence, the asertion (A) is true.
Protons and neutrons each have a mass of about 1 atomic mass unit (amu). Electrons have a mass approximately 1/1836 that of a proton, extremely small and usually neglected in atomic mass calculations. Hence reason (R) is true and reason (R) is the correct explanation of assertion (A).
Answered By
3 Likes
Related Questions
Select the correct statement(s) for the diagram shown below.
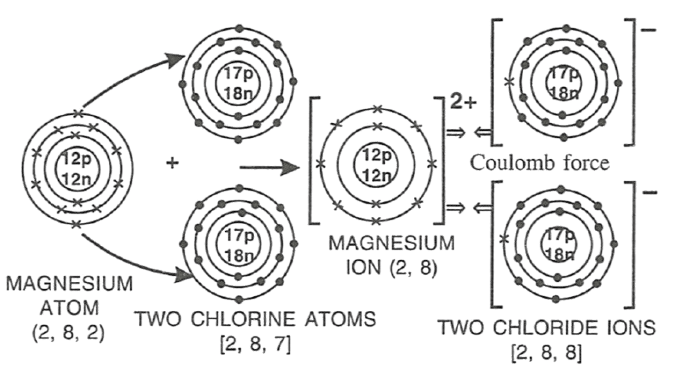
P — Mg looses electrons and undergoes reduction.
Q — Cl gains electrons and undergoes reduction.
R — Mg looses electrons and undergoes oxidation.
- Only P
- Only Q
- Only R
- Both Q and R
Select the correct statement(s) for the diagram shown below.
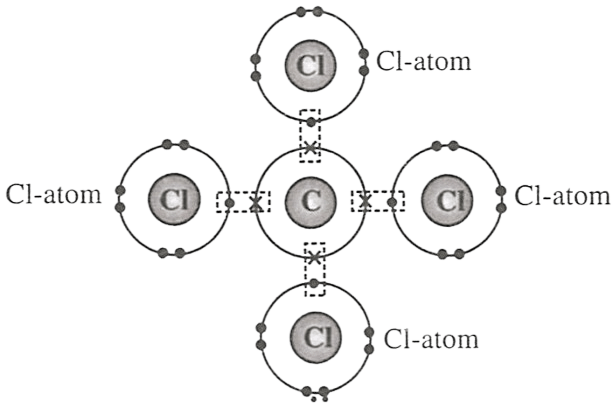
P — One atom of carbon transfers one electron to each chlorine atom.
Q — One atom of carbon shares four electron pairs, one with each of the four atoms of chlorine.
R — Carbon atom attains neon configuration and chlorine attains argon configuration after the combination.
- Only P
- Only Q
- Only R
- Both Q and R
Assertion (A): An alpha particle is the same as a Helium nucleus.
Reason (R): An alpha particle does not have any electron.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): Isotopes of an element have similar chemical properties.
Reason (R): Isotopes of an element have the same atomic number.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.