Chemistry
Select the correct statement(s) for the diagram shown below.
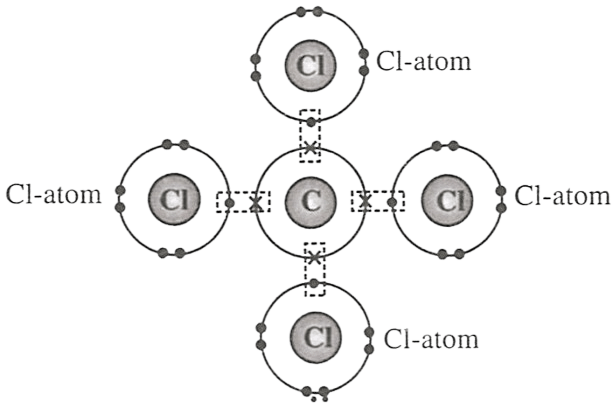
P — One atom of carbon transfers one electron to each chlorine atom.
Q — One atom of carbon shares four electron pairs, one with each of the four atoms of chlorine.
R — Carbon atom attains neon configuration and chlorine attains argon configuration after the combination.
- Only P
- Only Q
- Only R
- Both Q and R
Atomic Structure
5 Likes
Answer
Both Q and R
Reason — Carbon has the electronic configuration of [2, 4] and Chlorine has 7 electrons in the third orbit so its electronic configuration is [2, 8, 7]. Carbon forms covalent bond by sharing four electron pairs, one with each of the four atoms of chlorine. After sharing of electrons in order to obtain stable octate configuration, carbon attains electronic configuration of neon as [2, 8] and chlorine attains electronic configuration of argon as [2, 8, 8].
Answered By
4 Likes
Related Questions
An atom is electrically neutral because :
P — Electrons, protons and neutrons are equal in number.
Q — Protons are equal to neutrons.
R — Protons are equal to electrons.
- Only P
- Only Q
- Only R
- Both Q and R
Select the correct statement(s) for the diagram shown below.
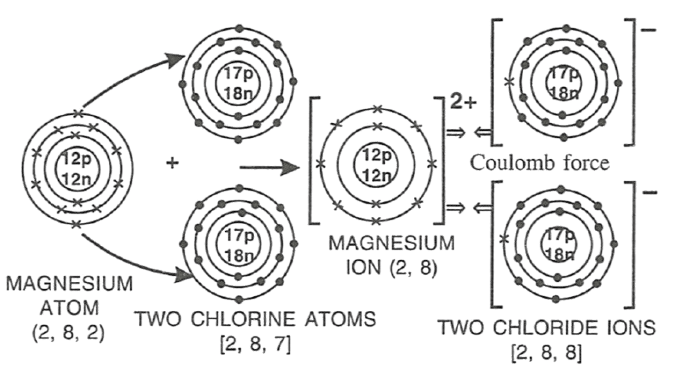
P — Mg looses electrons and undergoes reduction.
Q — Cl gains electrons and undergoes reduction.
R — Mg looses electrons and undergoes oxidation.
- Only P
- Only Q
- Only R
- Both Q and R
Assertion (A): The atomic mass of an element is mainly the mass of its nucleus.
Reason (R): The nucleus of an element contains neutrons and protons and the electrons have a negligible mass.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): An alpha particle is the same as a Helium nucleus.
Reason (R): An alpha particle does not have any electron.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.