Chemistry
An atom is electrically neutral because :
P — Electrons, protons and neutrons are equal in number.
Q — Protons are equal to neutrons.
R — Protons are equal to electrons.
- Only P
- Only Q
- Only R
- Both Q and R
Atomic Structure
3 Likes
Answer
Only R
Reason — An atom as a whole is electrically neutral, because the total positive charge of the nucleus is balanced by the total negative charge of the electrons, i.e., the number of protons and electrons in an atom are equal.
Answered By
1 Like
Related Questions
An atom is neutral, its nucleus contains:
P — Negatively charged particles.
Q — Neutral particles.
R — Positively charged particles.
- Only P
- Only Q
- Only R
- Both Q and R
Postulates of modern atomic theory are:
P — Atoms of an elements may not be alike.
Q — Atoms of elements combine in small whole numbers to form molecules.
R — Atoms can neither be created nor destroyed.
- Only P
- Only Q
- Only R
- Both P and Q
Select the correct statement(s) for the diagram shown below.
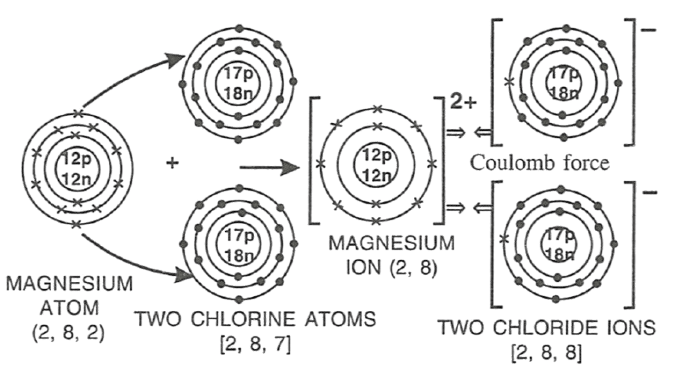
P — Mg looses electrons and undergoes reduction.
Q — Cl gains electrons and undergoes reduction.
R — Mg looses electrons and undergoes oxidation.
- Only P
- Only Q
- Only R
- Both Q and R
Select the correct statement(s) for the diagram shown below.
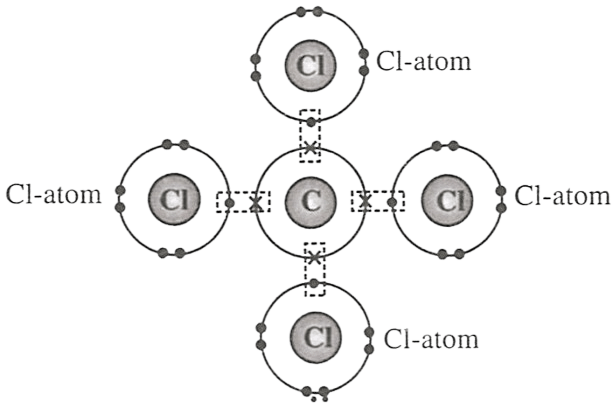
P — One atom of carbon transfers one electron to each chlorine atom.
Q — One atom of carbon shares four electron pairs, one with each of the four atoms of chlorine.
R — Carbon atom attains neon configuration and chlorine attains argon configuration after the combination.
- Only P
- Only Q
- Only R
- Both Q and R