Chemistry
Postulates of modern atomic theory are:
P — Atoms of an elements may not be alike.
Q — Atoms of elements combine in small whole numbers to form molecules.
R — Atoms can neither be created nor destroyed.
- Only P
- Only Q
- Only R
- Both P and Q
Atomic Structure
8 Likes
Answer
Only P
Reason — According to mordern atomic theory, atoms of an element may not be alike in all respects, as is seen in the case of isotopes.
Modern atomic theory states that atoms can be created and destroyed by nuclear fusion and fission and in the formation of organic compounds, the number of atoms that combine is very big such as thousands. Hence, other two statements are incorrect.
Answered By
3 Likes
Related Questions
An element Y has 2 electrons in the 2nd orbit and X has 7 electrons in its 3rd orbit. The compound formed between them has the formula:
- XY2
- YX2
- YX
- Y7X2
An atom is neutral, its nucleus contains:
P — Negatively charged particles.
Q — Neutral particles.
R — Positively charged particles.
- Only P
- Only Q
- Only R
- Both Q and R
An atom is electrically neutral because :
P — Electrons, protons and neutrons are equal in number.
Q — Protons are equal to neutrons.
R — Protons are equal to electrons.
- Only P
- Only Q
- Only R
- Both Q and R
Select the correct statement(s) for the diagram shown below.
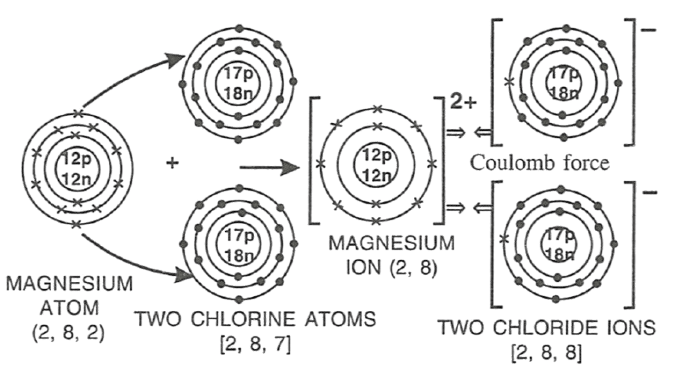
P — Mg looses electrons and undergoes reduction.
Q — Cl gains electrons and undergoes reduction.
R — Mg looses electrons and undergoes oxidation.
- Only P
- Only Q
- Only R
- Both Q and R