Chemistry
Assertion (A): Inflating a balloon seems to violate Boyle's law.
Reason (R): Boyle's law is valid for a fixed mass. Here, the mass of gas is changing.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Gas Laws
5 Likes
Answer
Both A and R are true and R is the correct explanation of A.
Explanation— When air is blown into a balloon, volume and pressure inside the balloon increase. Here, Boyle's law is not violated as the law is valid for a definite mass, whereas mass increases when more air is blown into the balloon. Hence, both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Answered By
2 Likes
Related Questions
Following figures illustrate graphical representations of gas laws. the figure which represents Charles' law is/are :
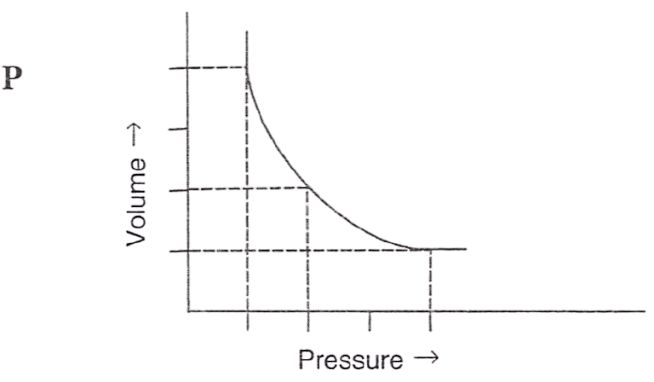
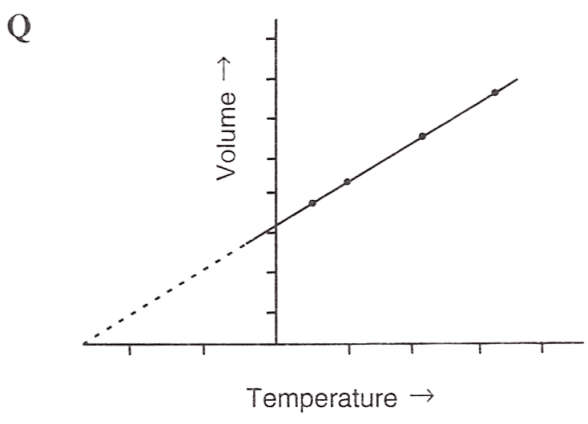
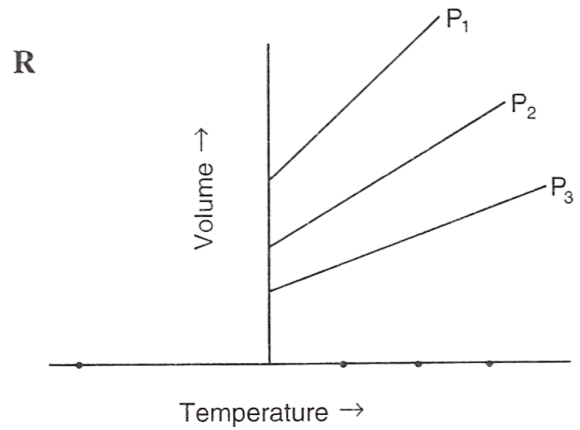
- Only P
- Only Q
- Only R
- Both Q and R
The term used for the graph of volume vs pressure for the verification of Boyle's law is :
P — Isobar
Q — Isotherm
R — Isotope
- Only P
- Only Q
- Only R
- Both P and Q
Assertion (A): The volume occupied by a gas is negligible at zero degree centigrade temperature.
Reason (R): Molecular motion ceases at 0 K.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): Gases exert pressure on the walls of the container they are kept in.
Reason (R): Gas molecules have a large kinetic energy. They strike the walls of the container in which they are kept with a certain force. The force per unit area is responsible for the pressure of the gas.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.