Chemistry
Assertion (A): A saturated solution becomes unsaturated on heating.
Reason (R): More amount of solute can dissolve in a solvent upon heating.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Water
8 Likes
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — When a saturated solution is heated to a higher temperature, then it becomes unsaturated. More solute can be dissolved in this solution now. Hence, both the assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Answered By
3 Likes
Related Questions
The figure shown below demonstrates the solubility curve of a substance. From the statements given below, choose which is/are correct :
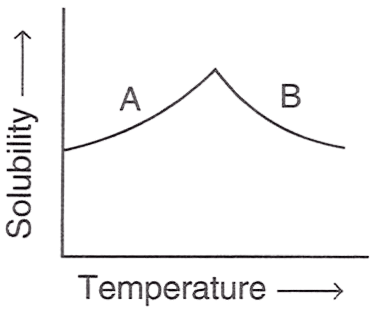
P — A is hydrated sodium chloride, B is anhydrous sodium chloride.
Q — A is Glauber's salt, B is sodium sulphate.
R — A is Gypsum, B is calcium sulphate.
- Only P
- Only Q
- Only R
- Both P and R
Assertion (A): Water is a universal solvent.
Reason (R): Water dissolves all substances except noble metals and glass.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): A white powder forms on the surface of washing soda crystals which are left exposed to the air.
Reason (R): Washing soda is a hygroscopic substance.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): A crusty 'boiler scale' is formed in boilers when hard water is used.
Reason (R): Hard water contains bicarbonates of calcium and magnesium.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.