Chemistry
Assertion (A): Zinc can displace copper from aqueous copper sulphate solution.
Reason (R): Copper is placed above zinc in the reactivity series.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Chemical Reaction
8 Likes
Answer
A is true but R is false.
Explanation — Zinc is more reactive than copper. So, zinc is placed above copper in reactivity series. Hence, zinc displaces copper from aqueous copper sulphate solution. Hence the assertion (A) is true.
CuSO4 (aq) + Zn → ZnSO4 + Cu ↓
Copper is less reactive than zinc and is placed below zinc in reactivity series. Less reactive elements cannot displace more active elements. Hence, copper cannot displace zinc from its salt solution. Hence the reason (R) is false.
Answered By
5 Likes
Related Questions
The figure given below demonstrates the preparation of oxygen.
2KClO3 (s) 2KCl (s) + 3O2 (g)
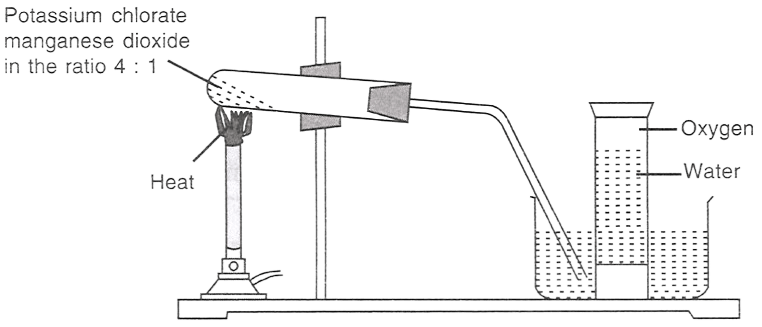
(I) Which of the following statements is/are correct about the reaction?
It is a decomposition reaction and is exothermic.
It is a decomposition reaction and is endothermic.
It is a photochemical decomposition reaction.
It is a combination reaction.
(II) Potassium chlorate is:
an oxidising agent.
a reducing agent.
both reducing as well as an oxidising agent.
all options are correct.
(III) In the reaction, manganese dioxide:
takes part in the reaction.
acts as a catalyst.
decreases the speed of reaction.
helps in producing more oxygen.
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
P — The temperature of the solution increases.
Q — The temperature of the solution decreases.
R — The temperature of the solution remains the same.
S — Salt formation takes place.
Only P
P and R
Q and R
P and S
Assertion (A): In a redox reaction, the electron gaining species acts as a reducing agent.
Reason (R): Oxidation and reduction reactions occur simultaneously in redox reaction.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): The decomposition of vegetable matter into compost is an endothermic reaction.
Reason (R): Decomposition reactions involve the breakdown of a single reactant into simpler products.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.