Chemistry
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
P — The temperature of the solution increases.
Q — The temperature of the solution decreases.
R — The temperature of the solution remains the same.
S — Salt formation takes place.
Only P
P and R
Q and R
P and S
Chemical Reaction
8 Likes
Answer
P and S
Reason — When a solution of an acid is mixed with a solution of a base, it forms salt and water. This is referred to as neutralization reaction. Neutralization reactions are also exothermic reactions. Hence the temperature of the solution increases.
For example :
NaOH + HCl ⟶ NaCl + H2O + Heat
Answered By
5 Likes
Related Questions
Aman performed the following four experiments
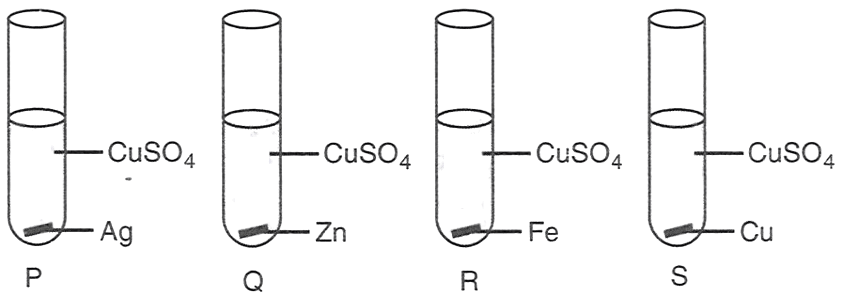
The experiments in which the blue colour of the solution will fade are :
P and Q
P, Q and R
Q and R
Q, R and S
The figure given below demonstrates the preparation of oxygen.
2KClO3 (s) 2KCl (s) + 3O2 (g)
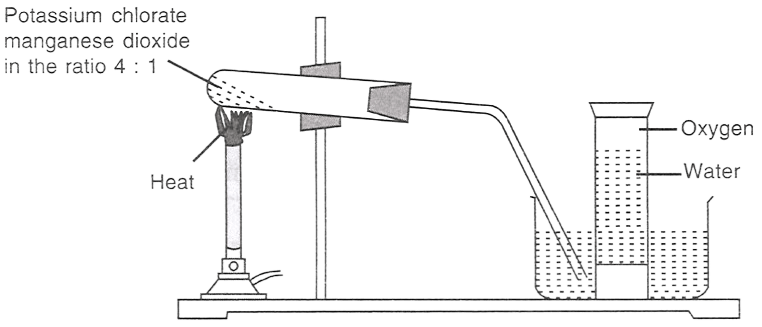
(I) Which of the following statements is/are correct about the reaction?
It is a decomposition reaction and is exothermic.
It is a decomposition reaction and is endothermic.
It is a photochemical decomposition reaction.
It is a combination reaction.
(II) Potassium chlorate is:
an oxidising agent.
a reducing agent.
both reducing as well as an oxidising agent.
all options are correct.
(III) In the reaction, manganese dioxide:
takes part in the reaction.
acts as a catalyst.
decreases the speed of reaction.
helps in producing more oxygen.
Assertion (A): Zinc can displace copper from aqueous copper sulphate solution.
Reason (R): Copper is placed above zinc in the reactivity series.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): In a redox reaction, the electron gaining species acts as a reducing agent.
Reason (R): Oxidation and reduction reactions occur simultaneously in redox reaction.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.