Physics
Astatine (At) is a radioactive element. Study the graph given below showing the number of protons vs the number of neutrons of radioactive nuclei.
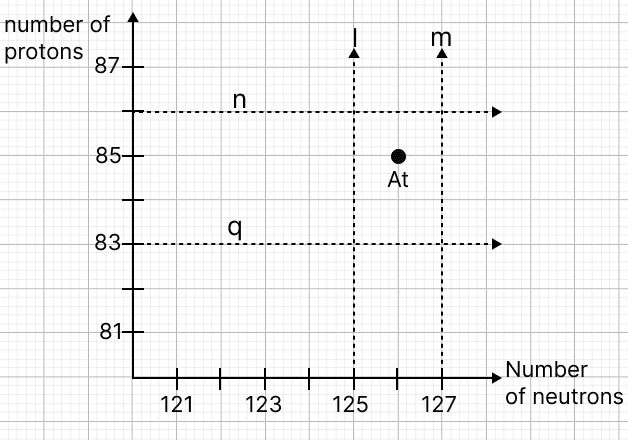
(a) Identify the mass number of the nucleus Astatine (At).
(b) Which line on the graph (l, m, n, or q) will never pass through the position of the daughter nuclei, regardless of any number of α, β, or γ emissions?
(c) Give a reason for your choice in (b).
Radioactivity
4 Likes
Answer
(a) From the graph,
Number of protons in Astatine = 85
Number of neutrons in Astatine = 126
Then,
Mass number of Astatine = Number of protons + Number of neutrons = 85 + 126 = 211
(b) The line m on the graph will never pass through the position of the daughter nuclei, regardless of any number of α, β, or γ emissions.
(c) As line m represents a daughter nucleus which has one excess neutron than Astatine and from α, β, or γ emissions, none has ability to increase the neutron number of Astatine and produce a daughter nucleus with one excess neutron so line m can not pass through any daughter nucleus of Astatine.
Answered By
2 Likes
Related Questions
The figure below shows a bicycle dynamo which is fitted to the tyre. When the wheels of the bicycle rotate, the spindle of dynamo attached to magnets rotate and the bulb glows.

(a) Name the phenomenon that takes place when the bulb glows while the person rides the bicycle.
(b) What will be the effect on the brightness of the bulb when the rider increases the speed of the bicycle?
Define background radiation. Give one internal source of this radiation.
(a) A coin lies at the bottom of a beaker. Water is poured into the beaker upto a height of 8 cm. Calculate the shift seen in the position of the coin.
(The refractive index of water is 4/3. The width of the glass wall of the beaker is negligible.)(b) How will the apparent depth be affected if the temperature of water is increased?
Draw a ray diagram to invert the image without deviation of light using right angle isosceles prism.