Chemistry
The atomic number of two atoms ‘X’ and ‘Y’ are 14 and 8 respectively.
State:
(a) the period to which ‘X’ belongs.
(b) the formula of the compound formed between ‘X’ and ‘Y’.
(Do not identify X and Y)
Periodic Table
15 Likes
Answer
(a) The atomic number of X is 14
Electronic configuration ⟶ 2, 8, 4
Number of electron shells = 3, hence X belongs to period 3
(b) The atomic number of Y is 8
Electronic configuration ⟶ 2, 6
X has a valency of 4 and Y has valency of 2
∴ Simplified formula of the compound is XY2
Answered By
9 Likes
Related Questions
Match the Column A with Column B:
Column A Column B (a) N2 + 3H2 ⇌ 2NH3 1. Vanadium Pentoxide (b) 4NH3 + 5O2 ⟶ 4NO + 6H2O 2. Nickel (c) 2SO2 + O2 ⇌ 2SO3 3. Iron (d) C2H4 + H2 ⟶ C2H6 4. Concentrated Sulphuric acid (e) CuSO4.5H2O ⟶ CuSO4 + 5H2O 5. Platinum (a) Draw the structural diagram for the following organic compounds:
- 2-methyl propene
- butanal
(b) Give IUPAC name for the following organic compounds:
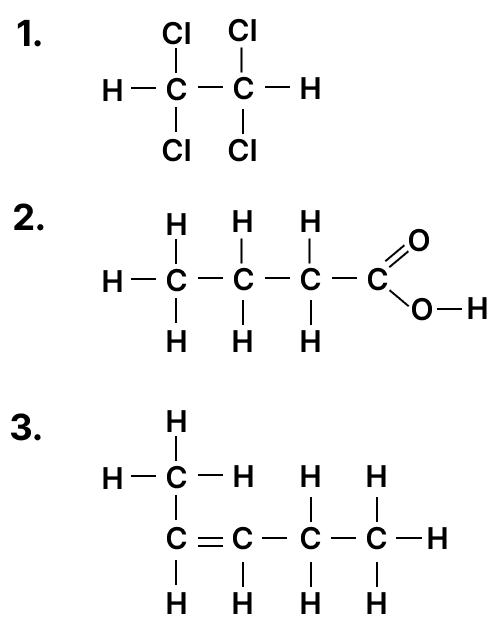
Justify the following statements:
(a) Anode is known as the oxidizing electrode.
(b) Graphite electrodes are preferred in the electrolysis of molten lead bromide.
The reaction between concentrated sulphuric acid and magnesium can be represented by the equation given below:
Mg + 2H2SO4 ⟶ MgSO4 + 2H2O + SO2
If 60 g of magnesium is used in the reaction, calculate the following:
(a) The mass of sulphuric acid needed for the reaction.
(b) The volume of sulphur dioxide gas liberated at S.T.P.
[Atomic weight: Mg=24, H=1, S=32, O=16]