Chemistry
Calcium oxide is a drying agent which removes water vapour. A student wanted to collect a dry sample of the hydrogen chloride gas produced. The student set up the apparatus as shown below but was unsuccessful in collecting any gas.
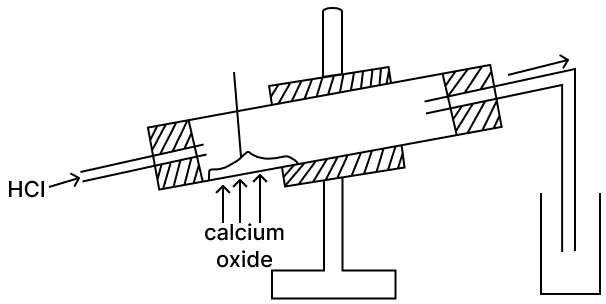
(a) What mistake did the student make?
(b) What change should be made by the student in order to collect the dry HCl gas?
Sulphuric Acid
4 Likes
Answer
(a) The student was unsuccessful in collecting any gas because calcium oxide (quick lime) react with hydrogen chloride.
CaO + 2HCl ⟶ CaCl2 + H2O
(b) The student can use conc. sulphuric acid as a drying agent. Conc. H2SO4 absorbs water vapour but does not react with HCl gas.
Answered By
3 Likes
Related Questions
Smith wrote the following statements incorrectly. Insert a word to correct the statements.
(a) Lead bromide conducts electricity.
(b) Copper reacts with nitric acid to form nitrogen dioxide gas.
(c) Bromoethane reacts with sodium hydroxide to produce ethanol and sodium bromide.
Match the Column A (showing the properties of H2SO4) with Column B (showing the reaction of H2SO4)
Column A
Properties of H2SO4Column B
Reaction of H2SO4(a) Acidic property 1. C12H22O11 + nH2SO4 ⟶ 12C + 11H2O + nH2SO4 (b) Dehydrating property 2. S + 2H2SO4 ⟶ 3SO2 + 2H2O (c) Non-volatile acid 3. CaO + H2SO4 ⟶ CaSO4 + H2O (d) Oxidizing agent 4. NaCl + H2SO4 ⟶ NaHSO4 + HCl Select the correct answer from the options given in the brackets:
(a) The ion which is discharged at the cathode during the electrolysis of CuSO4 solution using copper electrodes. [Cu2+, OH-, SO42-, H+]
(b) During electroplating of an article with Ag using sodium argentocyanide as an electrolyte, the anode is made of. [Cu, Ag, Pt, Na]
Ethane C2H6 burns in oxygen to produce carbon dioxide and water as shown in the equation given below:
2C2H6 + 7O2 ⟶ 4CO2 + 6H2O
Calculate the composition of the resulting gaseous mixture at room temperature when 60 c.c. of ethane burns in 250 c.c. of oxygen.