Chemistry
Complete the following sentences by choosing the correct word(s) from the brackets:
(a) …………… solution forms a coloured precipitate with ammonium hydroxide which is soluble in excess of ammonium hydroxide. [Ferrous chloride / Copper nitrate]
(b) Zinc blende is converted to zinc oxide by ……………. [Calcination / Roasting]
(c) …………… conducts electricity by the movement of ions. [Molten iron / Molten sodium chloride]
(d) The reaction that takes place at the anode during the electrolysis of aqueous Sodium argentocyanide with silver electrodes is ……………. [Ag ⟶ Ag+ + e-/ Ag+ + e- ⟶ Ag]
(e) The salt formed when ZnO reacts with hot concentrated NaOH is …………… . [sodium zincate / zinc hydroxide]
Analytical Chemistry
14 Likes
Answer
(a) Copper nitrate solution forms a coloured precipitate with ammonium hydroxide which is soluble in excess of ammonium hydroxide.
(b) Zinc blende is converted to zinc oxide by roasting.
(c) Molten sodium chloride conducts electricity by the movement of ions.
(d) The reaction that takes place at the anode during the electrolysis of aqueous Sodium argentocyanide with silver electrodes is Ag ⟶ Ag+ + e-
(e) The salt formed when ZnO reacts with hot concentrated NaOH is sodium zincate.
Answered By
9 Likes
Related Questions
A student was instructed by the teacher to prepare and collect ammonia gas in the laboratory by using aluminium nitride. The student had set up the apparatus as shown in the diagram below. Study the given diagram and answer the following questions:
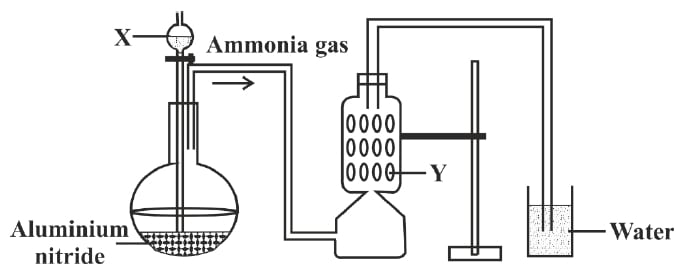
(a) Name the substance X added through the thistle funnel by the student.
(b) Write a balanced equation for the reaction occurring between Aluminium nitride and substance X.
(c) Identify the substance Y.
(d) State the function of Y.
(e) Why could the student not collect ammonia gas at the end of the experiment?
State the terms for the following:
(a) Undistilled alcohol containing a large amount of methanol.
(b) A salt formed by the partial replacement of the hydroxyl group of a di-acidic or a tri-acidic base by an acid radical.
(c) Organic compounds having the same molecular formula but different structural formula.
(d) The tendency of an atom to attract the shared pair of electrons towards itself when combined in a compound.
(e) The type of covalent bond in which electrons are shared unequally between the combining atoms.
Match the Column A with Column B:
Column A Column B (a) N2 + 3H2 ⇌ 2NH3 1. Vanadium Pentoxide (b) 4NH3 + 5O2 ⟶ 4NO + 6H2O 2. Nickel (c) 2SO2 + O2 ⇌ 2SO3 3. Iron (d) C2H4 + H2 ⟶ C2H6 4. Concentrated Sulphuric acid (e) CuSO4.5H2O ⟶ CuSO4 + 5H2O 5. Platinum (a) Draw the structural diagram for the following organic compounds:
- 2-methyl propene
- butanal
(b) Give IUPAC name for the following organic compounds: