Chemistry
The diagram given below shows the bonding in the covalent molecule AB2.
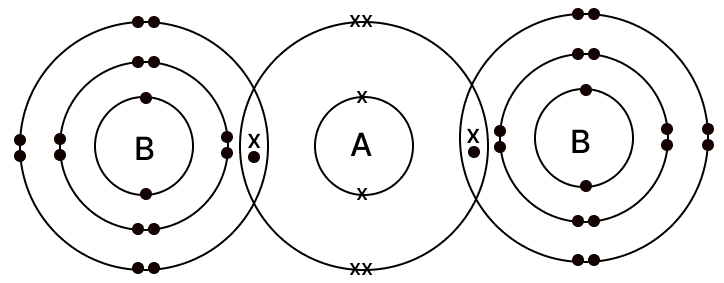
Which option represents the correct electronic configuration of atoms A and B before combining together to form the above molecule?
| A | B | |
|---|---|---|
| 1 | 2, 4 | 2, 8, 6 |
| 2 | 2, 4 | 2, 8, 7 |
| 3 | 2, 8 | 2, 8, 8 |
| 4 | 2, 6 | 2, 8, 7 |
Chemical Bonding
16 Likes
Answer
| 4 | 2, 6 | 2, 8, 7 |
Reason
- Atom A has six valence electrons (configuration 2, 6) and therefore needs two more electrons to complete its octet.
- Each atom B has seven valence electrons (configuration 2, 8, 7) and needs one additional electron to attain a stable, noble-gas configuration.
- In the AB2 molecule, atom A forms a single covalent bond with each of two B atoms. Through this sharing, atom A gains two electrons (one from each B), while each B gains one electron from A, so all three atoms achieve an octet.
Answered By
7 Likes
Related Questions
Assertion (A): The tendency of losing electrons increases down the Group.
Reason (R): The most reactive metal is placed at the top of Group 1.
- Both (A) and (R) are true, and (R) is the correct explanation of (A).
- Both (A) and (R) are true, and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
The ore that can be concentrated by using magnetic separation:
- Corundum
- Haematite
- Calamine
- Bauxite
Which of the following options has all the compounds which are members of the same homologous series?
- CH4, C2H6, C3H8
- CH4, C2H6, C3H6
- C3H4, C3H6, C3H8
- C2H4, C3H6, C4H10
Assertion (A): In the Contact Process SO3 gas is not directly dissolved in water to obtain sulphuric acid.
Reason (R): Dense fog or misty droplets of sulphuric acid are formed which is difficult to condense.
- Both (A) and (R) are true, and (R) is the correct explanation of (A).
- Both (A) and (R) are true, and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.