Chemistry
Ethane C2H6 burns in oxygen to produce carbon dioxide and water as shown in the equation given below:
2C2H6 + 7O2 ⟶ 4CO2 + 6H2O
Calculate the composition of the resulting gaseous mixture at room temperature when 60 c.c. of ethane burns in 250 c.c. of oxygen.
Related Questions
Calcium oxide is a drying agent which removes water vapour. A student wanted to collect a dry sample of the hydrogen chloride gas produced. The student set up the apparatus as shown below but was unsuccessful in collecting any gas.
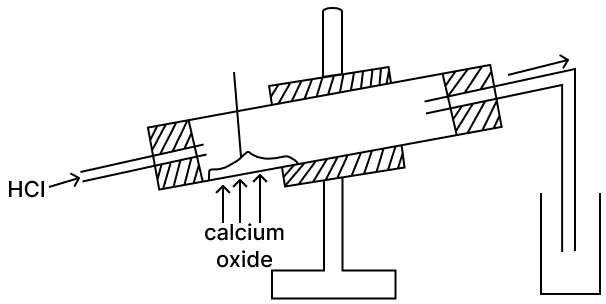
(a) What mistake did the student make?
(b) What change should be made by the student in order to collect the dry HCl gas?
Select the correct answer from the options given in the brackets:
(a) The ion which is discharged at the cathode during the electrolysis of CuSO4 solution using copper electrodes. [Cu2+, OH-, SO42-, H+]
(b) During electroplating of an article with Ag using sodium argentocyanide as an electrolyte, the anode is made of. [Cu, Ag, Pt, Na]
Match the uses of alloys in List 1 with the appropriate answer from List 2.
List 1 List 2 (a) Used in making decorative articles. 1. Stainless steel (b) An alloy used in making aircraft and light tools. 2. Brass (c) Used in making surgical Instruments. 3. Duralumin You are provided with some compounds in the box.
[SO2, PbO, CO, K2SO4, H2SO4, CH3COOH, NaHSO4, KCl]
Choose the compound from the above box that fits the descriptions from (a) to (c).
(a) An acid present in vinegar.
(b) An oxide which dissolves in water forming an acid.
(c) A salt formed by the incomplete neutralization of an acid by a base.