Chemistry
Which of the following statements is/are correct with respect to the figure shown alongside?
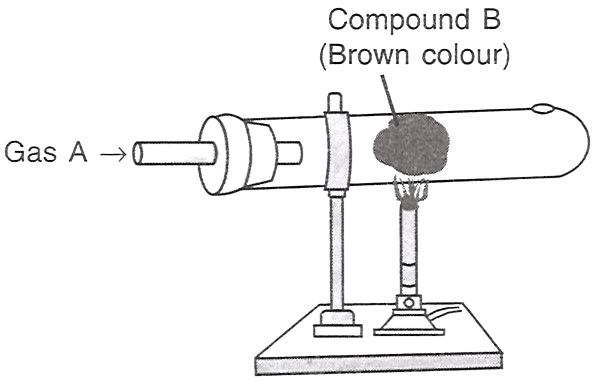
P — A is HCl, B is iron.
Q — A is Cl2, B is copper.
R — A is Cl2, B is iron.
Only P
Only Q
Only R
Both P and R
Answer
Only R
Reason — When chlorine gas (Gas A) is passed over heated iron (Compound B), it forms Iron(III) chloride, which is brown in color.
Whereas hydrochloric acid (HCl) will not produce the same reaction, and copper reacting with chlorine gas will produce copper(II) chloride, which is typically green or blue-green, not brown.
Hence A is chlorine gas and B is iron.
Related Questions
The reaction of neutralization is a :
- Displacement reaction
- Double decomposition
- Combination reaction
- Combination reaction between two compounds
FeS + H2SO4 ⟶ FeSO4 + H2S is an example of:
- Displacement reaction
- Double decomposition reaction
- Decomposition reaction
- Combination reaction
Aman performed the following four experiments
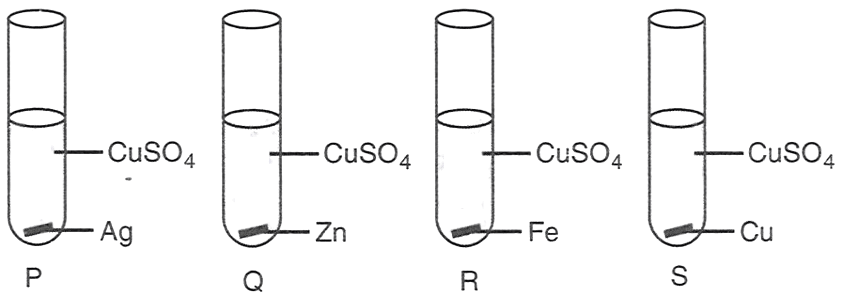
The experiments in which the blue colour of the solution will fade are :
P and Q
P, Q and R
Q and R
Q, R and S