Physics
The graph (Fig. A) illustrates the correlation between the number of protons (x-axis) and the number of neutrons (y-axis) for elements A, B, C, D, and E in the periodic table. These elements are denoted by the letters rather than their conventional symbols.
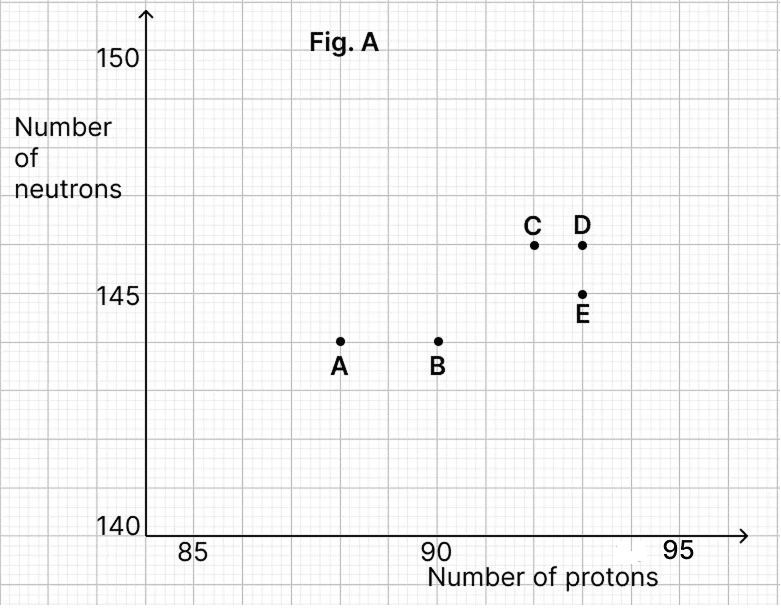
(a) Identify the radioactive radiation emitted when element C decays into element E. Represent this using a nuclear reaction.
(b) What is the special name given to elements D and E?
(c) If element C transforms into element B by emitting a radioactive ray, how will this ray behave in an electric field?
Radioactivity
7 Likes
Answer
(a) From the graph,
Number of proton in C = 92
Number of neutron in C = 146
Mass number of C = Number of proton in C + Number of neutron in C = 92 + 146 = 238
Number of proton in E = 93
Number of neutron in E = 145
Mass number of E = Number of proton in E + Number of neutron in E = 93 + 145 = 238
Since element C has one excess proton and one neutron less than the element E so if C decays into E then it's proton number increases by one and neutron number decreases by one keeping the mass number same which is only in case of beta radiation.
Thus, reaction will as follow :
23892C → 23893E + 0-1β
(b) As points D and E are situated on the same vertical line but on different horizontal lines it means they have same number of protons but different number of neutrons i.e., their atomic number is same but mass number is different so they are isotope of each other.
(c) Number of protons in B = 90
Number of neutron in B = 144
Mass number of B = Number of proton in B + Number of neutron in B = 90 + 144 = 234
As, element B has two protons less than element C and has mass number four less than C during a decay of C into B two protons and two neutrons emit which is nothing but an alpha particle.
Alpha particles shift towards negative plate in an electric field.
Answered By
5 Likes
Related Questions
An appliance with a metal covering, rated at 2 kW, 220 V, is to be connected in a circuit. Given below are four diagrams (P, Q, R & S) depicting different circuit configurations.

(a) Identify the safest circuit.
(b) Write two reasons, supported by mathematical calculations, where applicable, to justify your choice.
A nichrome wire X with length (l) & cross-sectional area (A) is connected to a 10 V source and another nichrome wire Y with length (2l) & cross-sectional area (A/2), is connected to a 20 V source.
(a) Compare the resistances of wires X and Y. [Given that the resistivity of nichrome is (ρ).]
(b) Compare the electrical power consumed by each wire.
(c) Compare the masses of these wires. (Given that the density of nichrome is d.)
(d) State True or False : Wire X and wire Y both show the same rise in temperature in the same time.
Three bulbs of powers P1, P2 and P3 (P1 < P2 < P3) are connected in a certain way that P3 glows brightest.
(a) What type of connection exists between these bulbs?
(b) Compare the voltage across these bulbs. (Use >, < or =)
(c) Will the circuit still function if one of the bulbs is fused?
The given diagram shows the output of an AC generator. If the speed of the generator coil is doubled, then:

(a) what is the effect on the physical quantity indicated by ‘a’?
(b) what is the effect on the physical quantity indicated as ‘b’?
(c) give reason for your answer in (b).