Chemistry
How is hydrogen manufactured? Describe with the equation(s) involved.
Hydrogen
59 Likes
Answer
Hydrogen is manufactured by Bosch process. It consists of following steps:
1. Production of water gas
Reactants : White hot coke and steam
Temperature : Around 1000°C
Process : Passage of steam over white hot coke [carbon]
Chamber : Specially designed convertor
2. Reduction of steam to hydrogen by carbon monoxide
Reactants : Water gas and excess steam
Temperature : Around 450°C
Catalysts : Iron [III] oxide [Fe2O3], promoter chromic oxide [Cr2O3]
Process : Excess steam is mixed with water gas, passed over a catalyst at elevated temperatures.
[CO is converted to CO2 with a further yield of hydrogen.]
3. Separation of carbon dioxide [CO2] and carbon monoxide from the above mixture
(a) CO2 is removed by dissolving mixture in water under pressure [30 atmospheres], or caustic potash solution to dissolve CO2.
2KOH + CO2 ⟶ K2CO3 + H2O
(b) CO is removed by dissolving mixture in ammoniacal cuprous chloride solution.
CuCl + CO + 2H2O ⟶ CuCl.CO.2H2O.
Thus, hydrogen gas is left behind.
Answered By
24 Likes
Related Questions
Which is the most preferred metal for the laboratory preparation of hydrogen. Why is any other metal not used?
Describe the laboratory preparation of hydrogen with a labelled diagram? How are the impurities removed.
Look at the following figure and answer the questions that follow:

(a) Which gas is prepared by this method marked as A.
(b) Name this method of collection. Why is this method used?
(c) Why is nitric acid not used as a reactant in the above method?
(d) Conc. H2SO4 is a good drying agent. However, it is not used here. Why?
Identify the gas evolved in the figure shown below.
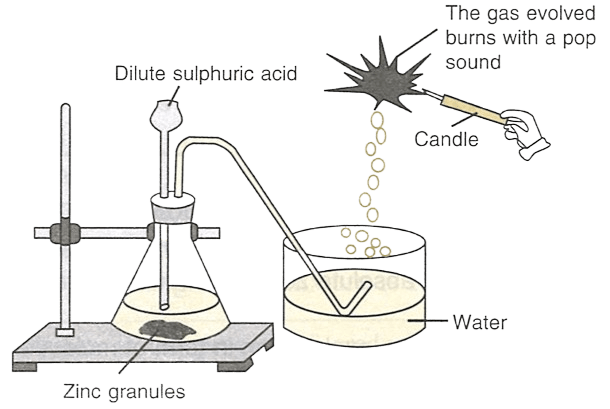
What would happen if the following changes are made:
(a) In place of zinc granules, same amount of zinc dust is taken in the test tube.
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) Sodium hydroxide is taken in place of dilute sulphuric acid and it is heated.