Chemistry
Look at the following figure and answer the questions that follow:

(a) Which gas is prepared by this method marked as A.
(b) Name this method of collection. Why is this method used?
(c) Why is nitric acid not used as a reactant in the above method?
(d) Conc. H2SO4 is a good drying agent. However, it is not used here. Why?
Hydrogen
65 Likes
Answer
(a) Hydrogen
(b) Downward displacement of water.
This method is used as:
- Hydrogen is virtually insoluble in water (20 ml of hydrogen dissolves in 1 litre of water under normal conditions)
- Hydrogen forms an explosive mixture with air and therefore, cannot be collected by downward displacement of air even though it is lighter than air.
(c) Nitric acid, even in its dilute form, is not used in the preparation of hydrogen from metals because it is a powerful oxidizing agent, and the oxygen formed due to its decomposition oxidizes the hydrogen to give water, thus defeating the purpose of the reaction.
3Zn + 8HNO3 ⟶ 3Zn(NO3)2 + 4H2O + 2NO↑
(d) Conc. H2SO4 is a good drying agent. However, it is not used to dry hydrogen as it reacts with hydrogen, thus defeating the purpose of the reaction.
H2SO4 + H2 ⟶ 2H2O + SO2
Answered By
41 Likes
Related Questions
Describe the laboratory preparation of hydrogen with a labelled diagram? How are the impurities removed.
How is hydrogen manufactured? Describe with the equation(s) involved.
Identify the gas evolved in the figure shown below.
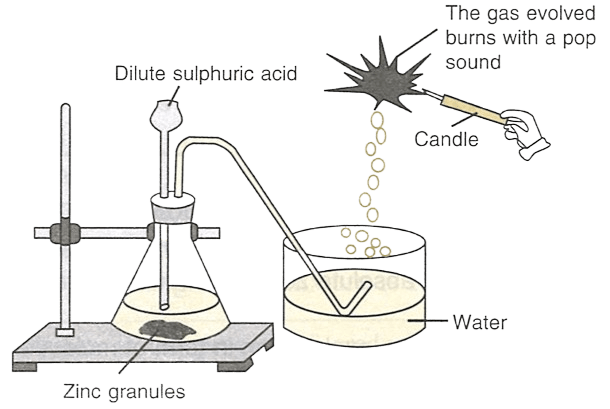
What would happen if the following changes are made:
(a) In place of zinc granules, same amount of zinc dust is taken in the test tube.
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) Sodium hydroxide is taken in place of dilute sulphuric acid and it is heated.
A metal is treated with dil. H2SO4. The gas evolved is collected by the method shown in the figure given below.
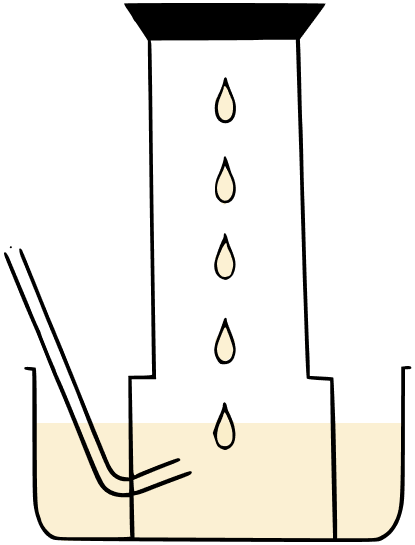
Answer the following:
(a) Name the gas.
(b) Name the method of collection of the gas.
(c) Is the gas soluble or insoluble in water?
(d) Is the gas lighter or heavier than air?
(e) Is the gas combustible or a supporter of combustion?