Science
Identify the type of reaction :
(a)
(b)
(c)
Chemical Reaction
1 Like
Answer
(a) Redox reaction.
Explanation — In this reaction, carbon reduces zinc oxide (ZnO) to zinc (Zn) while itself gets oxidized to carbon monoxide (CO). Hence, both oxidation and reduction occur simultaneously, which characterizes a redox process.
(b) Decomposition reaction.
Explanation — In this reaction, zinc carbonate (ZnCO3) breaks down on heating to form zinc oxide (ZnO) and carbon dioxide (CO2). It is also an endothermic reaction because heat energy is absorbed during the decomposition process.
(c) Combination reaction.
Explanation — In this reaction, magnesium (Mg) combines with oxygen (O2) to form magnesium oxide (MgO). It is also an exothermic reaction because a large amount of heat and light is released during the formation of magnesium oxide.
Answered By
1 Like
Related Questions
The domes of many building in Europe are made of copper. These domes now appear greenish in colour.
(a) Why do the domes appear greenish though copper is orange-red in colour?
(b) In your opinion, should the copper domes be replaced by iron domes to overcome the problem of change of colour of copper domes?
(c) Domes used to be made from thin sheets of metals. Why did the ancient architects use copper to make domes?
Amrita electrolysed distilled water using the set-up shown in figure 1. She was expecting two gases to be evolved at the anode and cathode respectively.

Suddenly, she realised that the bulb in the circuit did not glow when she used distilled water (figure 2)
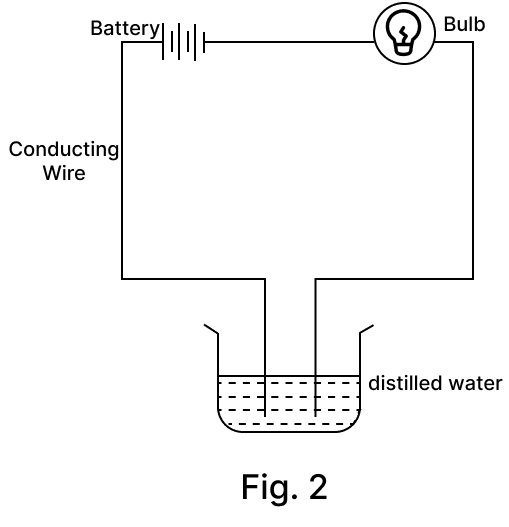
After this realization, she added a substance to the distilled water for electrolysis to take place.
Answer the following questions based on the information given above:
(a) Which gas was she expecting to be formed at the anode and which one at the cathode respectively?
(b) Why did the bulb not glow when Amrita passed electricity through distilled water?
(c) Which substance was added by Amrita to distilled water to get the expected result?
Sara took 2 mL of dilute NaOH solution in a test tube and added two drops of phenolphthalein solution to it. The solution turned pink in colour. She added dilute H2SO4 to the above solution drop by drop until the solution in the test tube became colourless. 40 drops of dilute H2SO4 were used for the change in colour from pink to colourless. When Sara added a drop of NaOH to the solution, the colour changed back to pink again.
Sara now tried the activity with different volumes of NaOH and recorded her observation in the table given below :S.
No.Volume of dil. NaOH taken (mL) Drops of dil. H2SO4 used 1 2 20 2 3 30 3 4 40 Answer the following questions based on the above information:
(a) If Sara used concentrated H2SO4 in place of dilute H2SO4, how many drops will be required for the change in colour to be observed?
- 40
- < 40
- > 40
Justify your answer.
(b) Sara measured 20 drops of dil. H2SO4 and found its volume to be 1 mL. If Sara observed a change in colour of NaOH solution by using 3 mL of H2SO4, how many mL of NaOH did she add to the test tube initially?
OR
Sara takes 10 drops of dilute H2SO4 in the test tube and adds two drops of phenolphthalein solution to it. Then she adds NaOH dropwise. Sara observes a change in colour after adding 20 drops of NaOH. What change in colour would she observe and why?
(c) Write a balanced chemical equation for the reaction taking place in the above experiment. Which of the following is true and why? The reaction is a
- neutralisation and double displacement reaction
- neutralisation and precipitation reaction
- precipitation and double displacement reaction
- neutralisation, double displacement as well as precipitation reaction.
A hydrocarbon with the formula CxHy undergoes complete combustion as shown in the following equation :
2CxHy + 9O2 → 6CO2 + 6H2O.
(a) What are the values of ‘x’ and ‘y’?
(b) Give the chemical (IUPAC) name of the hydrocarbon.
(c) Draw its electron dot structure.
(d) Name the alcohol which on heating with conc. H2SO4 will produce the above hydrocarbon CxHy.
(e) Write a balanced chemical equation for the reaction of CxHy with hydrogen gas in presence of Nickel.