Chemistry
(a) What do you mean by an acid salt ?
(b) Define the term 'Electronegativity'.
(c) State what do you see when a basic gas is passed over heated copper oxide.
(d) Write a balanced equation for the above reaction in (c).
(e) Draw an electron dot diagram of the negative ion formed when a basic gas is dissolved in water.
Acids Bases Salts
3 Likes
Answer
(a) Acid salts are the salts formed by partial replacement of the replaceable hydrogen ion of an acid molecule by a basic radical [metallic or ammonium ion]. Examples — Sodium Hydrogen Sulphate [NaHSO4], Disodium Hydrogen Phosphate [Na2HPO4]
(b) The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is called its electronegativity.
(c) Ammonia is a basic gas and a strong reducing agent. It reduces the black Copper [II] oxide to reddish brown Copper with the evolution of nitrogen gas.
(d) 2NH3 + 3CuO ⟶ 3Cu + 3H2O + N2 [g]
(e) The ion formed is Hydroxyl ion. Its electron dot diagram is shown below:
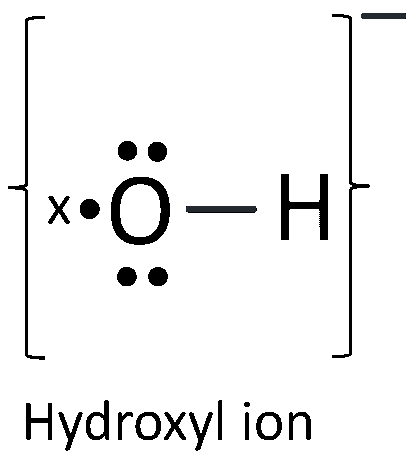
Answered By
2 Likes
Related Questions
Atom Y has 2 electrons in its N shell, Atom X has 7 electrons in its M shell and atom Z has 6 protons in its neutral state.
Answer the following questions:
(a) Which atom is likely to form a cation ?
(b) What is the formula of the compound formed between X and Z?
(c) Draw the electron dot diagram of the compound of X and Y ?
(d) State 2 properties of the compound formed between X and Z
From the list of substances, choose one substance in each case which matches the descriptions given below:
(ethene, ammonia, acetylene, ammonium chloride, nitric oxide, copper nitrate, water, sodium nitrate)
(a) A compound with two lone pairs.
(b) A nitrate which does not produce a brown gas on heating.
(c) A covalent compound which produces ions when dissolved in water.
(d) A salt which does not contain a metal ion.
(e) An unsaturated hydrocarbon with a triple bond between carbon atoms.
Complete the following table :
Types of organic compound Formula of the first member Chemical reaction with bromine (a) Alkane (b) Alkene (c) Alkyne C2H2 (a) State the type of bonding of the oxide of the element 'A' having electronic configuration 2, 8, 8. 1.
(b) Write the formula of the oxide of A.