Chemistry
An organic compound ‘X’ contains carbon, oxygen and hydrogen only. The percentage of carbon and hydrogen are 47.4% and 10.5% respectively. The relative molecular mass of ‘X’ is 76. Find the empirical formula and the molecular formula of ‘X’.
[Atomic weight: C = 12, O = 16, H = 1]
Related Questions
Given below is a diagram showing the placement of five different oxides. With respect to the given diagram answer the following questions:
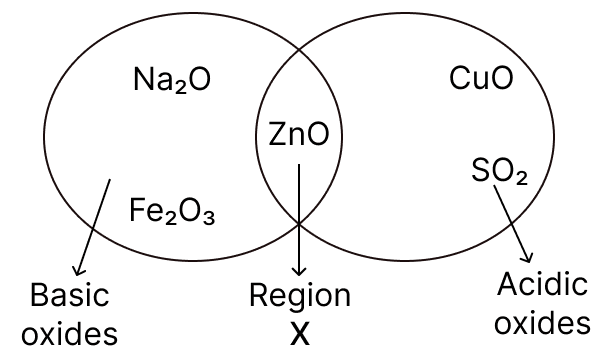
(a) Name the type of oxide represented in region X in the diagram.
(b) Identify the oxide which has been incorrectly placed in the above diagram.
(c) Name the oxide from the above diagram which will form an alkali when dissolved in water.
Given below are organic compounds labelled A to F. Answer the questions that follow:
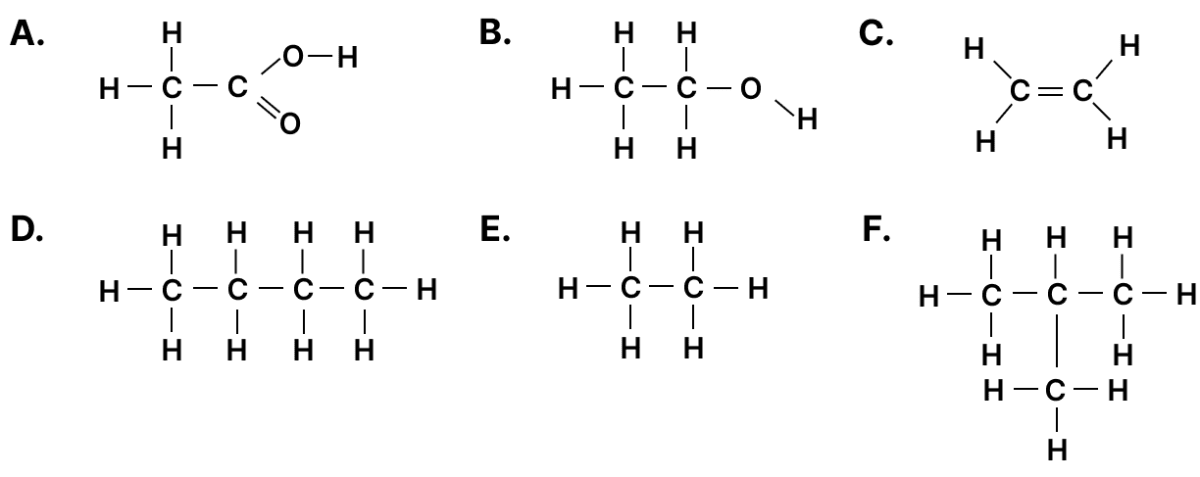
(a) Which compound forms a single product with bromine?
(b) Which two compounds have the same molecular formula?
(c) Which two compounds will react together in the presence of concentrated H2SO4 to form a product with a fruity smell?
Seema added a few pieces of copper turnings to a test tube containing concentrated acid P and she noticed that a reddish-brown gas evolved.
(a) Name the acid P used by Seema.
(b) Write a balanced chemical equation for the reaction that took place.
Answer the following questions with reference to the concentration of bauxite ore.
(a) Name the process used to concentrate the ore.
(b) Give a balanced chemical equation for the conversion of aluminium hydroxide to pure alumina.