Chemistry
Seema added a few pieces of copper turnings to a test tube containing concentrated acid P and she noticed that a reddish-brown gas evolved.
(a) Name the acid P used by Seema.
(b) Write a balanced chemical equation for the reaction that took place.
Nitric Acid
6 Likes
Answer
(a) The acid P is concentrated nitric acid (HNO3).
(b) Cu + 4HNO3 ⟶ Cu(NO3)2 + 2H2O + 2NO2
Answered By
5 Likes
Related Questions
Given below are organic compounds labelled A to F. Answer the questions that follow:
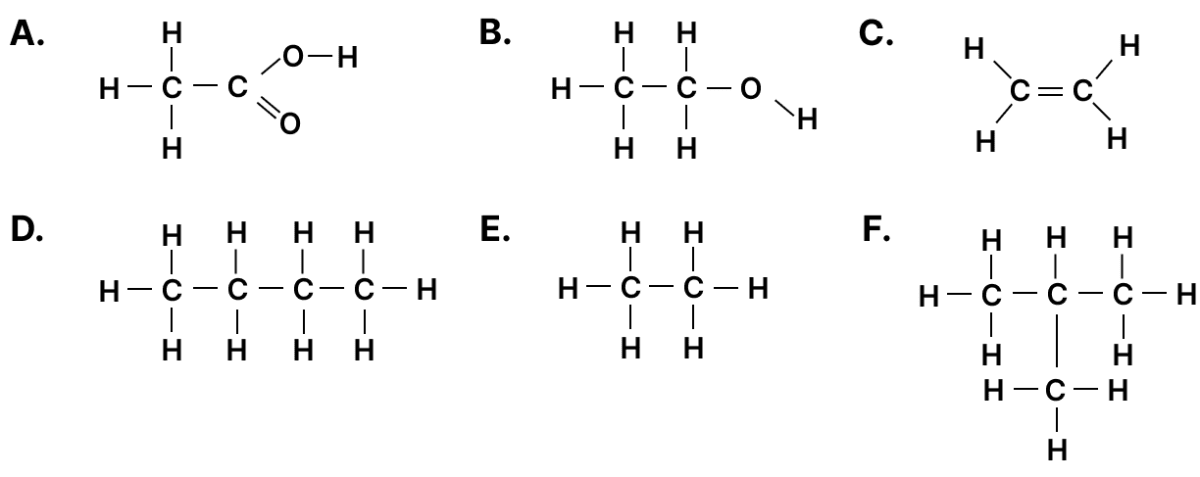
(a) Which compound forms a single product with bromine?
(b) Which two compounds have the same molecular formula?
(c) Which two compounds will react together in the presence of concentrated H2SO4 to form a product with a fruity smell?
An organic compound ‘X’ contains carbon, oxygen and hydrogen only. The percentage of carbon and hydrogen are 47.4% and 10.5% respectively. The relative molecular mass of ‘X’ is 76. Find the empirical formula and the molecular formula of ‘X’.
[Atomic weight: C = 12, O = 16, H = 1]Answer the following questions with reference to the concentration of bauxite ore.
(a) Name the process used to concentrate the ore.
(b) Give a balanced chemical equation for the conversion of aluminium hydroxide to pure alumina.
Draw the dot and cross structure of the following:
(a) An ionic compound formed when Mg reacts with the dilute HCl.
(b) A covalent compound formed when H2 reacts with Cl2.
(c) The positive ion produced when ammonia gas is dissolved in water.
[Atomic number: Mg = 12, Cl = 17, H = 1, N = 7]