Physics
A solid of mass 60 g at 100°C is placed in 150 g of water at 20°C. The final steady temperature is 25°C. Calculate the heat capacity of solid.
[Specific heat capacity of water = 4.2 Jg⁻¹K⁻¹]
Related Questions
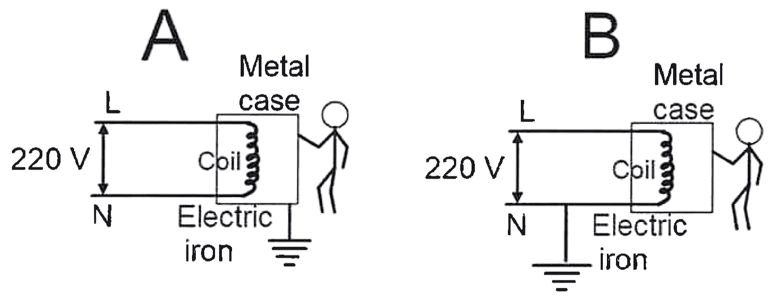
If live wire makes an accidental contact with the metal case, which circuit (A or B) in the diagram, illustrating an electric iron, is considered safe for the user (Assuming the fuse is present in the live wire in both circuits)? Justify your answer.
A transformer is used to change a high alternating e.m.f. to a low alternating e.m.f. of the same frequency.
(a) Identify the type of transformer used for the above purpose.
(b) State whether the turns ratio of the above transformer is = 1 or > 1 or < 1.
(a) Name the principle of AC generator.
(b) State its one use.
(a) Name the radiations that are emitted during the decay of a nucleus, which has highest penetrating power?
(b) Does the emission of the above-mentioned radiation result in a change in the mass number?