Chemistry
State one observation for each of the following:
(i) Concentrated nitric acid is reacted with sulphur.
(ii) Ammonia gas is passed over heated copper (II) oxide.
(iii) Copper sulphate solution is electrolysed using copper electrodes.
(iv) A small piece of zinc is added to dilute hydrochloric acid.
(v) Lead nitrate is heated strongly in a test tube.
Answer
(i) Reddish brown nitrogen dioxide gas is evolved.
S + 6HNO3 [conc.] ⟶ H2SO4 + 2H2O + 6NO2
(ii) Black copper [II] oxide is reduced to brown copper.
2NH3 + 3CuO ⟶ 3Cu + 3H2O + N2 [g]
(iii) A brownish pink deposit of copper metal is seen at the cathode when copper solution is electrolyzed using copper electrodes. The blue colour of Copper Sulphate solution does not fade and Copper anode diminishes in mass.
(iv) Effervescence of H2 gas can be seen.
Zn + 2HCl ⟶ ZnCl2 + H2
(v) White precipitate of PbCl2 is formed which is soluble in hot water.
Pb(NO3)2 + 2HCl ⟶ PbCl2 + 2HNO3
Related Questions
Fill in the blanks with the choices given in brackets:
(i) Conversion of ethanol to ethene by the action of concentrated sulphuric acid is an example of …………… (dehydration/dehydrogenation/dehydrohalogenation)
(ii) When sodium chloride is heated with concentrated sulphuric acid below 200°C, one of the products formed is …………… (sodium hydrogen sulphate / sodium sulphate / chlorine)
(iii) Ammonia reacts with excess chlorine to form …………… (nitrogen / nitrogen trichloride / ammonium chloride)
(iv) Substitution reactions are characteristic reactions of …………… (alkynes / alkenes / alkanes)
(v) In Period 3, the most metallic element is …………… (sodium / magnesium / aluminium)
Write a balanced chemical equation for each of the following reactions:
(i) Reduction of copper (II) oxide by hydrogen.
(ii) Action of dilute sulphuric acid on sodium hydroxide.
(iii) Action of dilute sulphuric acid on zinc sulphide.
(iv) Ammonium hydroxide is added to ferrous sulphate solution.
(v) Chlorine gas is reacted with ethene.
(i) Calculate:
The number of moles in 12g of oxygen gas. [O = 16]
The weight of 1022 atoms of carbon.
[C = 12, Avogadro’s No. = 6 x 1023]
(ii) Molecular formula of a compound is C6H18O3. Find its empirical formula.
(i) Give the IUPAC name of the following organic compounds:
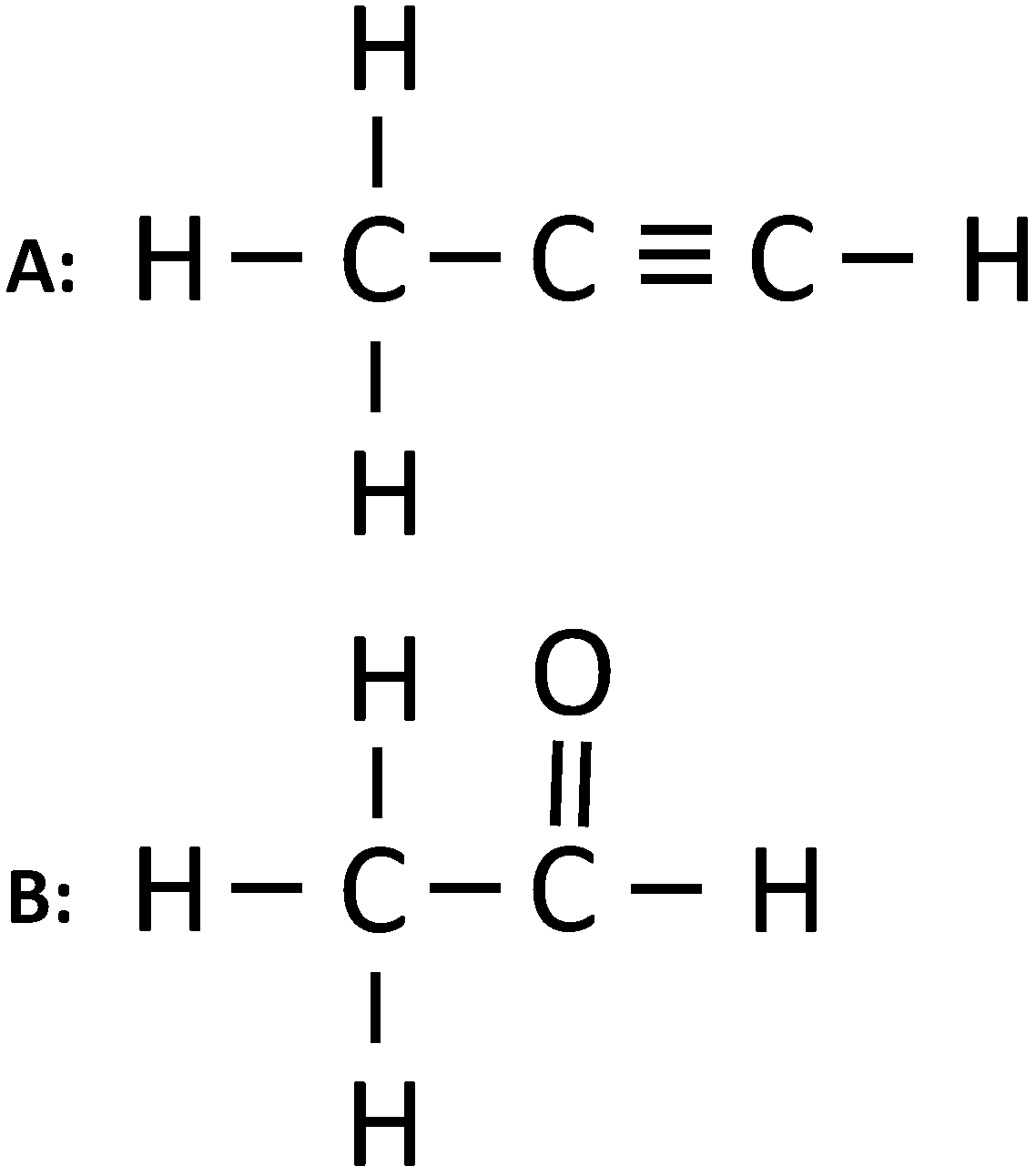
(ii) What is the special feature of the structure of ethyne
(iii) Name the saturated hydrocarbon containing two carbon atoms.
(iv) Give the structural formula of acetic acid.