Chemistry
STP is called standard temperature and pressure. The standard temperature and standard pressure respectively are :
- 273 K and 760 mm
- 0°C and 760 cm
- 273°C and 1 atmosphere
- 373 K and 76 cm
Gas Laws
35 Likes
Answer
273 K and 760 mm
Reason — The standard values chosen are 0°C or 273 K for temperature and 1 atm or 760 mm of Hg for pressure. These values are known as standard temperature and pressure (STP).
Answered By
24 Likes
Related Questions
'The volume of a given amount of a gas is directly proportional to its absolute temperature at a constant pressure' is the statement of :
- Gay-Lussac's law
- Boyle's law
- Charles' law
- Mendeleev's law
The volume of a given mass of a gas at constant temperature and 10 atmospheric pressure is 10 litres. Its volume at 5 atmospheres will be :
- 5 litres
- 10 litres
- 15 litres
- 20 litres
Following figures illustrate graphical representations of gas laws. the figure which represents Charles' law is/are :
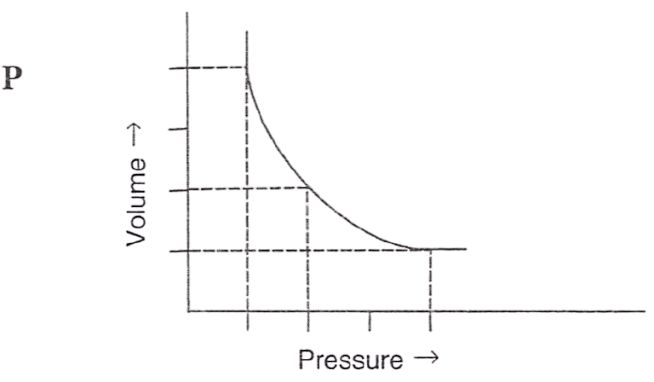
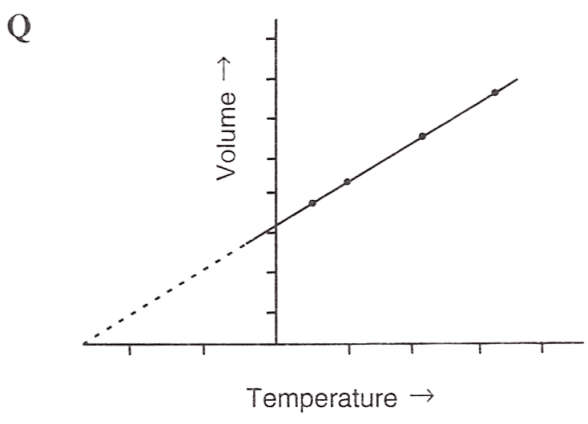
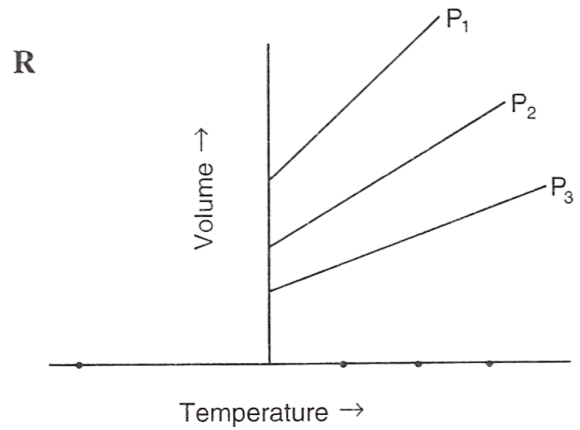
- Only P
- Only Q
- Only R
- Both Q and R
The term used for the graph of volume vs pressure for the verification of Boyle's law is :
P — Isobar
Q — Isotherm
R — Isotope
- Only P
- Only Q
- Only R
- Both P and Q