Chemistry
Three different solutions X (sodium chloride solution), Y (acetic acid) and Z (sugar solution) were used for electrolysis by a student. When the circuit was completed, he noticed that the bulb glowed in the electrolytic cell containing:
- X & Y
- Y & Z
- Z & X
- X, Y & Z
Answer
X & Y
Reason — X (NaCl solution) is an electrolyte, it contains free Na+ and Cl- ions, so it conducts electricity well.
Y (acetic acid) is a weak electrolyte, it partially ionises to give H+ and CH3COO- , so it can conduct where the bulb may glow dimmer than with a strong electrolyte. Whereas, Z (sugar solution) contains molecular sucrose which does not dissociate into ions.
Related Questions
The gas evolved in the diagrammatic set up given below turns calcium hydroxide solution milky. The gas evolved is:
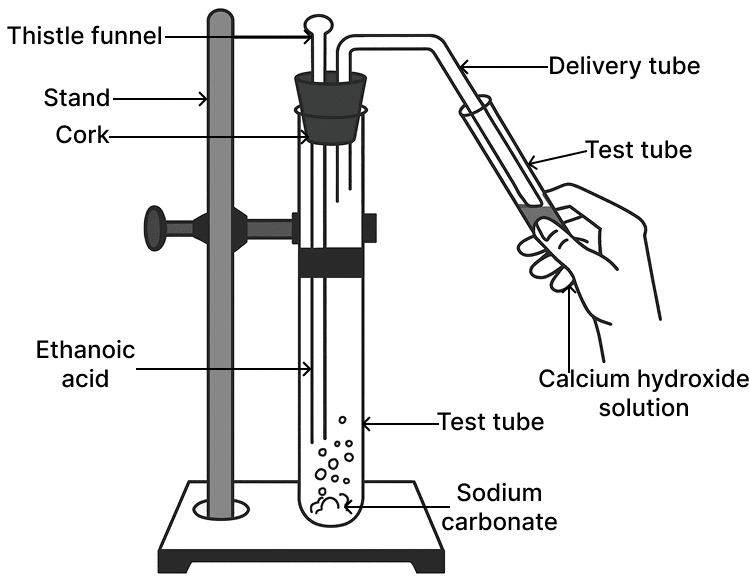
- CH4
- C2H6
- CO2
- SO2
Which gas is evolved when ammonia gas is passed over buff yellow PbO?
- N2O
- NO
- N2
- NO2
An element X has an electronic configuration 2, 2. The compound formed when X combines with oxygen is most likely to be:
- a compound with a low melting point.
- a gas that dissolves in water to form an electrolyte.
- a good conductor in both solid and molten state.
- an ionic solid.
If an element has a low ionisation potential, it is most likely to be a:
- metal
- non-metal
- metalloid
- inert gas