Chemistry
What do you understand by redox reactions ? Explain oxidation and reduction in terms of loss or gain of electrons.
Chemical Bonding
90 Likes
Answer
Redox Reaction — A reaction in which oxidation and reduction occur simultaneously is called an oxidation-reduction reaction or redox reaction.
In redox reaction there is a transfer of electrons between two atoms.
The reaction in which electron(s) is gained is called a reduction reaction and the reaction in which there is a loss of electron(s) is called an oxidation reaction.
Answered By
56 Likes
Related Questions
Draw an electron dot diagram of the structure of — hydronium ion. State the type of bonding present in it.
By drawing an electron dot diagram, show the lone pair effect leading to the formation of — ammonium ion from ammonia gas and hydrogen ion.
Study the extract of the Periodic table given below and answer the questions that follow. Give the alphabet corresponding to the element in question: DO NOT repeat an element.
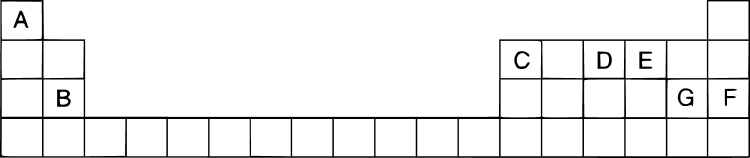
(a) Which element forms an electrovalent compound with G?
(b) The ion of which element will migrate towards the cathode during electrolysis?
(c) Which non metallic element has the valency 2?
(d) Which is the inert gas?
The following table shows the electronic configuration of the elements W, X, Y, Z :
Element W X Y Z Electronic
Configurations2, 8, 1 2, 8, 7 2, 5 1 Answer the following questions based on the table above:
(i) What type of bond is formed between:
- W and X
- Y and Z
(ii) What is the formula of the compound formed between :
- X and Z
- W and X