Chemistry
X, Y & Z are three metallic atoms in successive order belonging to the same group such that atomic radii of ‘X’ is the smallest. Which of the three atoms is the best reducing agent?
- X
- Y
- Z
- All three have the same reducing power.
Periodic Table
13 Likes
Answer
Z
Reason — The greater the tendency to lose electrons, the greater the metallic character and the stronger the reducing power of a metal. As we move down a group, atomic size increases and ionisation energy decreases, so the tendency to lose electrons and thus the reducing power also increases. Therefore, of the three atoms, Z (the lowest member of the group) is the best reducing agent.
Answered By
7 Likes
Related Questions
Which pair of reactants can be best used to produce lead (II) sulphate?
- Sulphuric acid + Lead
- Sulphuric acid + Lead hydroxide
- Sodium sulphate + Lead nitrate
- Potassium sulphate + Lead oxide
Aqueous copper (II) sulphate is electrolysed using copper electrodes. Which statement about the electrolysis is not correct?
- An oxidation reaction occurs at the positive electrode.
- The current is carried through the electrolyte by ions.
- The positive electrode loses mass.
- The number of copper (II) ions in the electrolyte decreases.
40 cm3 of methane (CH4) is reacted with 60 cm3 of oxygen. The equation for the reaction is given below:
CH4 + 2O2 ⟶ CO2 + 2H2O
All volumes are measured at room temperature. What is the total volume of the gases remaining at the end of the reaction?
- 60 cm3
- 40 cm3
- 45 cm3
- 50 cm3
A student was instructed by the teacher to prepare and collect ammonia gas in the laboratory by using aluminium nitride. The student had set up the apparatus as shown in the diagram below. Study the given diagram and answer the following questions:
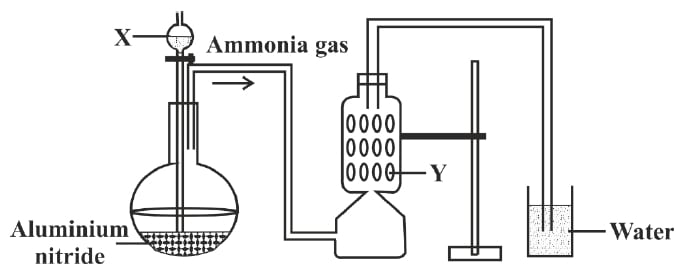
(a) Name the substance X added through the thistle funnel by the student.
(b) Write a balanced equation for the reaction occurring between Aluminium nitride and substance X.
(c) Identify the substance Y.
(d) State the function of Y.
(e) Why could the student not collect ammonia gas at the end of the experiment?