What is the state of ice?
- Liquid
- Gas
- Solid
Answer
Solid
Reason — Ice is water in its frozen form, where water molecules are arranged in a rigid, closely packed structure which is held together by strong intermolecular forces, keeping the molecules in fixed positions, giving ice a definite shape and volume — characteristics of a solid state.
Ice will become ............... if kept in open.
- Water
- Solid
- Gas
Answer
Water
Reason — When ice is kept in the open at temperatures above 0°C , it absorbs heat from its surroundings which breaks the strong intermolecular forces between water molecules, changing ice from a solid to liquid water which is a process called melting.
If the melted ice cubes are heated, then they get converted into steam by the process of
- Freezing
- Evaporation
- Condensation
Answer
Evaporation
Reason — When the melted ice (liquid water) is heated, it absorbs heat energy which increases the kinetic energy of water molecules until they escape into the air as water vapour (steam) below their boiling point. This change from liquid to gas is called evaporation.
Which of the following has a fixed shape?
- Water
- Steam
- Ice
Answer
Ice
Reason — Ice is a solid, and solids have a fixed shape and volume because their particles are tightly packed in a rigid structure whereas water (liquid) and steam (gas) take the shape of their container.
When the ice cubes melt, is there any change in their volume?
- Yes
- No
- Can't say
Answer
Yes
Reason — Ice has less closely packed structure of molecules than water so ice takes up more space and when it melts, this structure collapses, and the molecules pack more closely, reducing the volume.
Why do ice cubes take on a particular shape?
Answer
When water freezes, it solidifies and adopts the exact shape of the space it’s in — usually the compartments of an ice tray. The rigid structure of ice then locks that shape in place so ice cubes take on a particular shape due to formation of stronger intermolecular forces when water freezes in a container.
What is the ideal temperature for freezing water to make ice cubes?
Answer
The ideal temperature for freezing water into ice cubes is at it's freezing point i.e., 0 °C since at 0 °C, water begins to change from liquid to solid, and keeping the temperature below this point ensures the freezing happens faster and the ice cubes form completely.
What happens when salt is added to ice?
Answer
When we add salt to ice, the ice starts melting faster because salt mixes with the thin layer of water present on the surface of the ice and makes it harder for the water to freeze again. As a result, the ice melts even if the temperature is below it's freezing point. This is why people put salt on icy roads in cold countries — it helps melt the ice and makes the roads less slippery.
Fill in the blanks.
(a) ............... is anything that has mass and occupies space.
(b) The particles of matter are called ............... .
(c) Molecules are constantly ............... in all possible directions.
(d) Molecules have ............... between them.
Answer
(a) Matter is anything that has mass and occupies space.
(b) The particles of matter are called molecules .
(c) Molecules are constantly moving in all possible directions.
(d) Molecules have space between them.
Fill in the blanks.
(a) Arrange solids, liquids and gases in order of increasing intermolecular force.
............... < ............... < ...............
(b) ............... have a fixed shape and size.
(c) ............... have a fixed volume but no fixed shape.
(d) ............... have neither fixed shape nor volume.
Answer
(a) Arrange solids, liquids and gases in order of increasing intermolecular force.
gases < liquids < solids
(b) Solids have a fixed shape and size.
(c) Liquids have a fixed volume but no fixed shape.
(d) Gases have neither fixed shape nor volume.
Fill in the blanks.
(a) A ............... is the smallest unit of matter that can exist independently.
(b) On cooling, a liquid gets changed into a ............... .
(c) The molecules in a ............... move freely in all directions.
(d) ............... have a definite volume.
(e) Gases are not ............... at all.
Answer
(a) A molecule is the smallest unit of matter that can exist independently.
(b) On cooling, a liquid gets changed into a solid .
(c) The molecules in a gas move freely in all directions.
(d) Solids and liquids have a definite volume.
(e) Gases are not rigid at all.
State whether the given statements are true (T) or false (F). Correct the false statements.
(a) The process by which a solid gets changed into a liquid is called boiling.
(b) The molecules of liquid are not fixed at their positions.
(c) Solids are hard and rigid.
(d) The intermolecular force in gases is very strong.
(e) The molecules of a liquid come closer and start vibrating in their fixed positions.
Answer
(a) False
Corrected Statement — The process by which a solid gets changed into a liquid is called melting.
(b) True
(c) True
(d) False
Corrected Statement — The intermolecular force in gases is very weak.
(e) False
Corrected Statement — The molecules of a solid come closer and start vibrating in their fixed positions.
Match the columns.
| Column A | Column B | ||
|---|---|---|---|
| 1. | Liquid to gas | (a) | Gas |
| 2. | Fixed shape and definite volume | (b) | Condensation |
| 3. | Gas to liquid | (c) | Liquid |
| 4. | Can be compressed | (d) | Boiling |
| 5. | Irregular shape and definite volume | (e) | Solid |
Answer
| Column A | Column B | ||
|---|---|---|---|
| 1. | Liquid to gas | (d) | Boiling |
| 2. | Fixed shape and definite volume | (e) | Solid |
| 3. | Gas to liquid | (b) | Condensation |
| 4. | Can be compressed | (a) | Gas |
| 5. | Irregular shape and definite volume | (c) | Liquid |
Which of the following is a solid?
- Air
- Water
- Chalk
- Milk
Answer
Chalk
Reason — Chalk has a fixed shape and volume, and its molecules are closely packed together with strong intermolecular forces of attraction between them. Hence, it is a solid. Water and milk are liquids and air is a gas.
Which of the following has negligible intermolecular space between its molecules?
- Gas
- Solid
- Liquid
- Atom
Answer
Solid
Reason — The molecules in a solid are tightly packed, i.e., the intermolecular distance among the molecules is the least whereas the molecules in a liquid are not very tightly packed, hence the intermolecular distance is moderate and the molecules in a gas are loosely packed, i.e., the intermolecular distance is the maximum.
The temperature at which a liquid gets converted into a solid is called ............... point.
- Boiling
- Condensation
- Melting
- Freezing
Answer
Freezing
Reason — The temperature at which a substance changes from its liquid state to its solid state on cooling is called freezing point.
Which of the following is not a characteristic of molecules of matter?
- Molecules have a very large size.
- Molecules are constantly moving.
- Molecules have spaces between them.
- Molecules attract each other.
Answer
Molecules have a very large size.
Reason — Characteristics of molecules :
- Molecules are very small in size.
- Molecules have spaces between them.
- Molecules are constantly moving.
- Molecules attract each other.
Which of the following has the negligible intermolecular force between its molecules?
- Solid
- Liquid
- Gas
- Atom
Answer
Gas
Reason — The intermolecular forces are strongest in solids, moderate in liquids and weakest in gases.
Write one word or few words for the following.
(a) The temperature on which a solid gets changed into a liquid
(b) It is formed by combining two or more atoms
(c) It can be beaten either into sheets or into powdered form
(d) The molecules are loosely packed in this state of matter
(e) It has neither a fixed shape nor a fixed volume
Answer
(a) Melting point
(b) Molecule
(c) Solid
(d) Gas
(e) Gas
Define the following terms.
(a) Intermolecular space
(b) Condensation
(c) Freezing point
(d) Intermolecular force of attraction
(e) Evaporation
Answer
(a) Intermolecular space — The space between the molecules of a substance is called the intermolecular space or intermolecular distance.
(b) Condensation — The process by which a substance changes from its gaseous state to its liquid state on cooling is called condensation.
(c) Freezing point — The temperature at which a substance changes from its liquid state to its solid state on cooling is called freezing point.
(d) Intermolecular force of attraction — The force of attraction between the molecules is known as intermolecular force of attraction.
(e) Evaporation — The process in which when a liquid is heated, some molecules escape from its surface below its boiling point is called evaporation.
Give reasons for the following statements.
(a) Solids are rigid and incompressible.
(b) Change in temperature cause changes in state of matter.
(c) Liquids take the shape of the container.
(d) Ice changes to water and water changes to steam.
(e) Water droplets form on a glass of cold water.
Answer
(a) In solids, molecules are closely packed and the intermolecular space between them is very less. The molecules remain fixed and vibrate about their mean positions. Hence, solids keep their shape rigid (fixed) and cannot be compressed into a smaller volume (incompressible).
(b) When a substance is heated, its molecules move faster and can break free from their fixed positions, changing from solid to liquid or from liquid to gas. When it is cooled, the molecules slow down and come closer, changing from gas to liquid or from liquid to solid.
(c) In liquids, the intermolecular forces are weaker than in solids. Hence, the molecules are not tightly packed and can slide past one another. This free movement of molecules allows liquids to flow and take the shape of the container in which they are kept
(d) When ice is heated, the molecules present in it start vibrating more and more until they start sliding over each other. Thus, ice changes to water. On heating the water further, the molecules start moving even faster until they become free to move in all directions. Thus, water changes to steam.
(e) The cold glass cools the air around it and the water vapour present in the air loses heat, changes into liquid water, and forms tiny droplets on the outer surface of the glass. This process is called condensation.
How does a liquid get converted into a gas?
Answer
When we heat a liquid, the molecules present in it start moving faster until they become free to move in all directions. Thus, a liquid gets changed into a gas.
Why do solids expand very little on heating?
Answer
Solids expand very little on heating because their particles are packed very closely together and are held in fixed positions by strong intermolecular forces and when it is heated, the molecules only vibrate a little faster and push slightly further apart, so the increase in size is very small compared to liquids or gases.
How does a solid get converted into a liquid?
Answer
When we heat a solid, the molecules present in it start vibrating more and more until they start sliding over each other. Thus, a solid gets changed into a liquid.
Why are gases very loosely packed?
Answer
The intermolecular force in gases is almost negligible due to this, the molecules in gases are very loosely packed.
Give three examples of each state of matter.
Answer
Following are three examples of each state of matter :
- Solid : Wood, spoon, ruler.
- Liquid : Water, milk, juice.
- Gas : A cylinder filled with LPG, a ballon filled with air, steam.
Write a note on matter.
Answer
Matter is anything that has mass and occupies space and is made up of very small particles called molecules. In matter, molecules are always in continuous motion which shows, molecules have space between them and are held together by intermolecular forces so due to difference in intermolecular forces and intermolecular space, matter has three different states i.e., solid, liquid and gas, at normal temperature.
Explain the changing states of matter by taking the example of water.
Answer
Water exists in three states i.e., ice (solid), water (liquid), and steam (gas) and it changes from one state to another when heat is added or removed.
Solid to liquid
Ice is the solid form of water and when heated, it absorbs heat energy which makes the molecules move faster and break free from their fixed positions and hence, melts into liquid water. This complete process is called melting.Liquid to gas
When water is heated, its particles move even faster and at boiling point it changes into steam (gas form).Gas to Liquid
When steam or water vapour is cooled, particles lose energy and come closer together to form water (liquid). This process is called condensation.Liquid to Solid
When water is cooled, its molecules slow down and arrange into a fixed position which is known as freezing process and thus, liquid water changes into ice.
Compare the characteristics of solids and liquids.
Answer
The differences between solids and liquids is given below :
| S. No. | Solids | Liquids |
|---|---|---|
| 1. | The molecules in a solid are tightly packed, i.e., the intermolecular distance among the molecules is the least. | The molecules in a liquid are not very tightly packed, hence the intermolecular distance is moderate. |
| 2. | The intermolecular forces are the strongest in solids. | The intermolecular forces are moderate in liquids. |
| 3. | Solids have a fixed shape and size, and possess a definite volume. | Liquids have a definite volume but not a fixed shape. |
| 4. | Solids are rigid. | Liquids are less rigid. |
| 5. | The molecules in a solid do not move, but only vibrate about their mean positions. | The molecules in a liquid can move in all directions but within the boundary of the liquid. |
Describe a simple activity to show that molecules have spaces between them.
Answer
The simple activity to show that molecules have spaces between them is given below :
Aim : To demonstrate the presence of spaces between particles of matter
Materials Required : A measuring cylinder (100 mL), a glass rod, powdered sugar, a spoon and water
Procedure :
- Take 100 mL water in a measuring cylinder and mark the level of water.
- Dissolve some powdered sugar in it with the help of a glass rod as shown below.

Observation : You will observe that even though the sugar has dissolved, the water level is same as before.
Conclusion : This activity shows that when sugar is dissolved in water, its molecules go into the spaces present between the water molecules. Due to this, there is no change in the water level when sugar is added to it. Hence, spaces are present between molecules of matter.
Write a note on composition of matter.
Answer
Matter is made up of very small particles called molecules and a molecule is the smallest unit of matter which can exist independently and retains all its physical and chemical properties. It is made up of various elements. An element is a substance which cannot be subdivided into two or more simpler substances by any means. Atoms are the smallest unit of an element which may or may not exist independently.
Harsh's mother asked him to close the knob of the gas stove after use, but he forgot. After some time, his mother observed a smell of rotten cabbages in the whole house. She went to the kitchen and saw that the knob was still open. She closed it immediately. Later, she made Harsh understand that it is very dangerous to keep the knob of the stove open, as it can catch fire.

(a) Which characteristic of gas is responsible for this smell in the whole house?
(b) Name the gases that a gas cylinder usually contains.
(c) Is it possible to keep solids and liquids compressed in a cylinder?
(d) What precautions should we follow to avoid any mishaps in the kitchen?
(e) What would you do in such a situation?
Answer
(a) Intermolecular force in gases is almost negligible due to which they move freely in all directions and occupy all the space available to them. Hence, the smell of gas spreads in the whole house.
(b) A domestic gas cylinder usually contains Liquefied Petroleum Gas (LPG).
(c) Solids and liquids cannot be compressed easily in a cylinder because their molecules have less space between them which does not allow them to compress.
(d) Precautions to avoid mishaps in the kitchen :
- Always check that the gas knob is closed after use.
- Keep the kitchen well-ventilated.
- Do not light a match or switch on electrical appliances if you smell gas.
- Get the gas pipeline and cylinder checked regularly for leaks.
(e) In such a situation, I would :
- Immediately close the gas knob and the cylinder valve.
- Open all windows and doors for ventilation.
- Avoid lighting any flames or using electrical switches.
- Inform an adult or the gas company if the smell continues.
Why can't liquids be compressed?
Answer
Liquids can’t be compressed easily because their particles are already packed very close together with very little empty space between them so when it is compressed, there’s hardly any space for the particles to move closer, so its volume doesn’t change much.
We can move our hands through air and water, but not through a wooden structure. Why?
Answer
We can move our hands through air and water because their particles are loosely packed with large spaces in between. The particles can easily move aside when our hand passes through. In wood, the particles are very tightly packed and fixed in position, making it hard and rigid. Hence, we cannot move our hands through a wooden structure.
In which of the following cases will the sugar dissolve in a glass of water faster, and why?
Case 1: Water in the glass is cold
Case 2: Water in the glass is warm
Answer
Warm water has larger intermolecular spaces because its particles move farther apart when heated. This gives more room for sugar particles to slip in and mix with the water. In cold water, the intermolecular spaces are smaller, so sugar dissolves more slowly.
What happens to the volume of a substance when it changes from a solid to a liquid?
Answer
For most substances, when they change from a solid to a liquid, their volume increases slightly because the particles move apart a little as they lose their fixed positions. But water is special as when ice (solid) melts into water (liquid), its volume actually decreases because water molecules pack more closely in the liquid state than in the solid state.
Can ice be changed directly into water vapour?
Answer
Yes, ice can change directly into water vapour without becoming liquid water first and this process is called sublimation where a solid changes directly into a gas.
A science experiment was conducted by a man in his kitchen. He kept some ice cubes at room temperature. Ice cubes melted into water. Then he placed this water in a pot on the stove and turned on the heat. As the water started to heat up, he observed some changes. As the temperature increased, he observed that the water started to form tiny bubbles and steam began to rise. Then, he placed a metal lid on top of the pot to cool down the steam. After a few minutes, when he removed the lid, he saw water droplets on the inner side of the lid.
He plotted the following graph. Observe the graph carefully and answer the following questions.
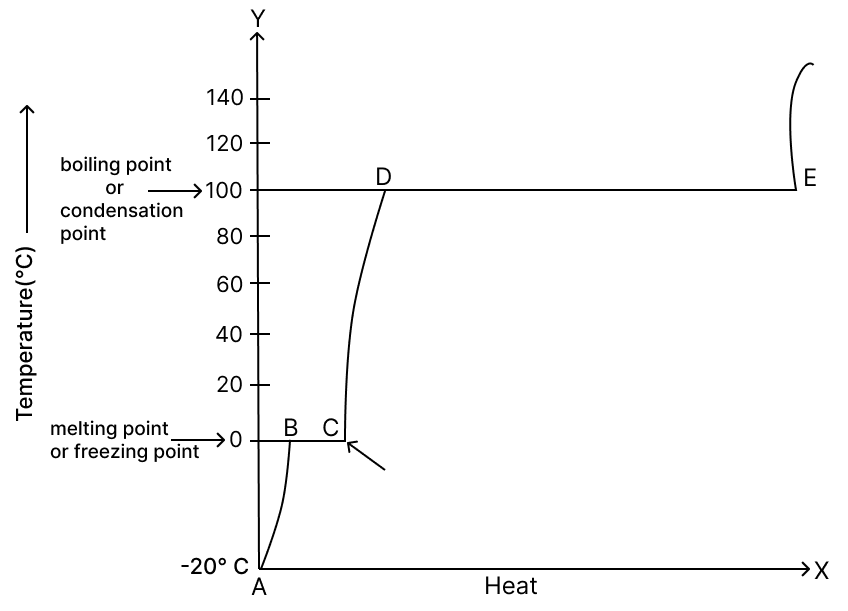
(a) What experiment did the man perform?
(b) Label A, B, C, D and E in the graph.
(c) Why did he observe water droplets on the lid?
(d) At what temperature does water change into ice?
(e) Why does it take so long to warm water in winter?
(f) Does the quantity of water also change with its state?
Answer
(a) The man performed an experiment to show the changes of state of water — solid (ice) → liquid (water) → gas (steam) → liquid again (condensation).
(b) Likely graph labels:
- A – Ice (solid) being heated (temperature rising from below - 20°C to 0°C)
- B – Melting point (0°C) – ice starts melting
- C – Water (liquid) being heated (temperature rising from 0°C to 100°C)
- D – Boiling point (100°C) – water starts boiling and turning to steam
- E – Steam (gas) being heated further above 100°C
(c) He observed water droplets on the lid because steam cooled down on touching the cold metal lid, changing from gas to liquid. This process is called condensation.
(d) Water changes into ice at 0°C (freezing point).
(e) It takes longer to warm water in winter because in winter, the starting temperature is lower so heat require to warm the water will be greater.
(f) No, the quantity (mass) of water does not change when it changes state. Only the arrangement and movement of molecules change.