Chemistry
A student performed an experiment to measure pressure and volume of a gas at constant temperature and noted the following:
| Pressure (mm of Hg) | Volume (cm3) |
|---|---|
| 100 | 80 |
| 125 | x |
| 200 | 40 |
| y | 32 |
Calculate the value of x and y. Which law was used in the calculations? Draw graphs to show:
(i) volume plotted against pressure.
(ii) PV plotted against pressure
Gas Laws
122 Likes
Answer
(a) V1 = Initial volume of the gas = 80 cm3
P1 = Initial pressure of the gas = 100 mm of Hg
P2 = Final pressure of the gas = 125 mm of Hg
V2 = Final volume = ?
By Boyle's Law:
Substituting the values :
∴ x = 64 cm3
(b) V1 = Initial volume of the gas = 40 cm3
P1 = Initial pressure of the gas = 200 mm of Hg
V2 = Final volume of the gas = 32 cm3
P2 = Final pressure of the gas = ?
By Boyle's Law:
Substituting the values :
∴ y = 250 mm of Hg
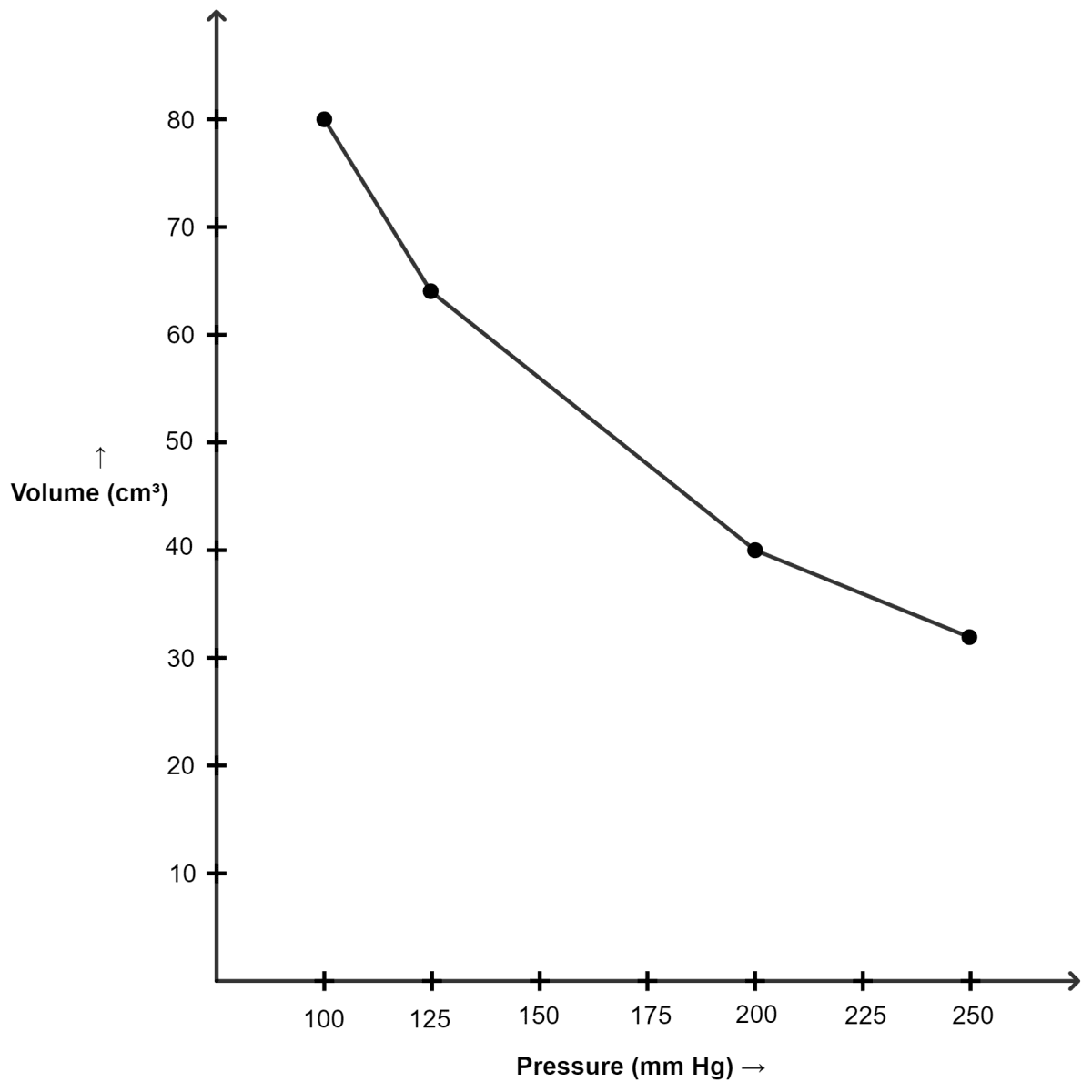
(i) Graph of volume plotted against pressure :
| Pressure (mm of Hg) | Volume (cm3) |
|---|---|
| 100 | 80 |
| 125 | 64 |
| 200 | 40 |
| 250 | 32 |
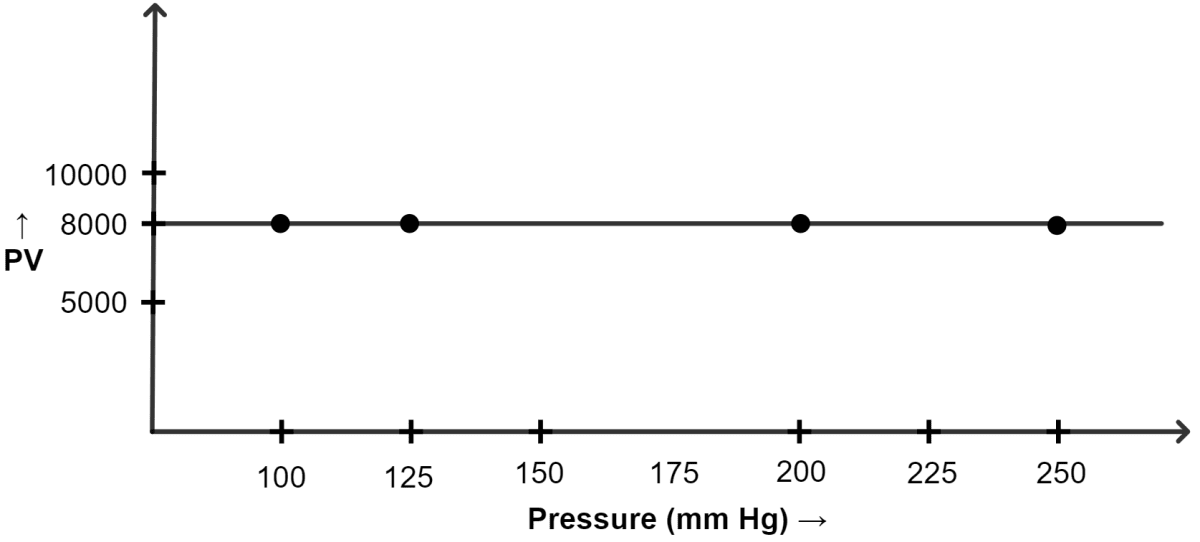
(ii) Graph of PV plotted against pressure :
| Pressure (mm of Hg) | Volume (cm3) | PV |
|---|---|---|
| 100 | 80 | 8000 |
| 125 | 64 | 8000 |
| 200 | 40 | 8000 |
| 250 | 32 | 8000 |
Answered By
58 Likes
Related Questions
Volume of certain amount of a gas at 25°C and 100 cm Hg pressure is 80 mL. The gas is expanded to 160 mL keeping the temperature constant. Calculate the pressure of the expanded gas.
At a particular temperature, a certain quantity of gas occupies a volume of 74 cm3 at a pressure of 760 mm. If the pressure is decreased to 740 mm, what will be the volume of the gas at the same temperature?
At a constant temperature, volume of a gas was found to be 400 cm3 at a pressure of 760 mm Hg. If the pressure of the gas is increased by 25%, find the new volume.
A vessel of capacity 600 cm3 contains hydrogen gas at a pressure of 330 cm Hg. What will be the pressure of hydrogen gas, when the vessel is connected to another vessel of 300 cm3 capacity ?