Chemistry
A student was asked to perform two experiments in the laboratory based on the instructions given:
Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
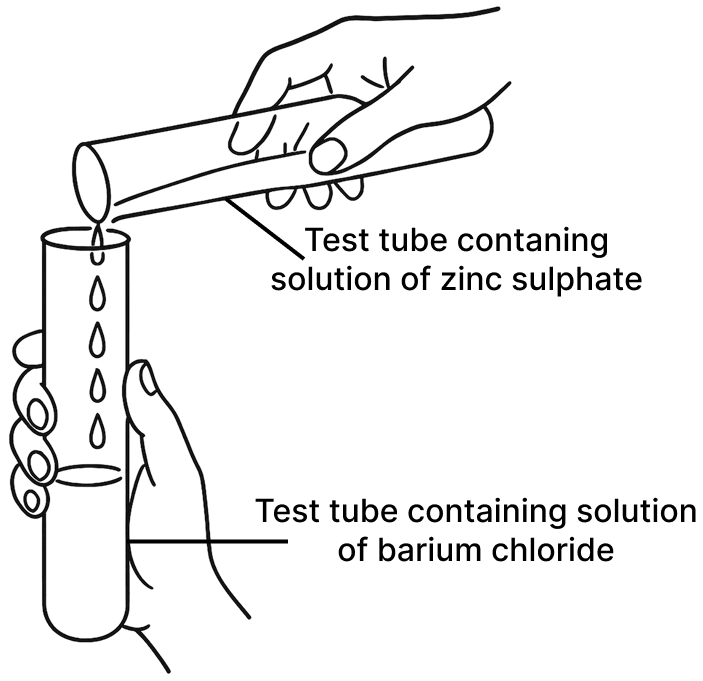
(a) Experiment 1
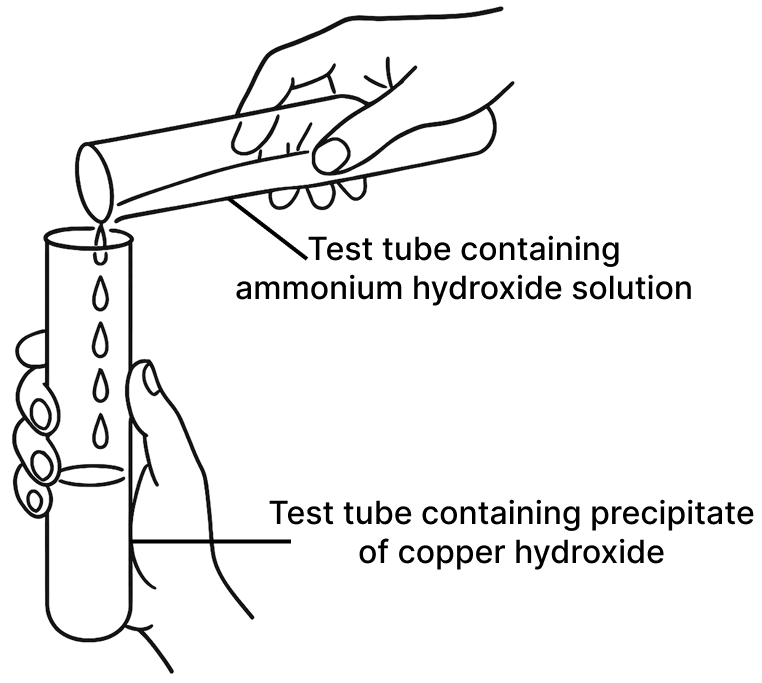
(b) Experiment 2
Answer
(a) White precipitate of barium sulphate is formed.
Reason — When a solution of zinc sulphate is added to a solution of barium chloride, a double displacement reaction occurs. This reaction involves the exchange of ions between the two compounds and a white precipitate of barium sulphate is formed.
ZnSO4 (aq) + BaCl2 (aq) ⟶ BaSO4 (s) + ZnCl2 (aq).
(b) Blue precipitate dissolves to form an inky blue/deep blue solution.
Reason — The light blue precipitate of copper(II) hydroxide (Cu(OH)2) dissolves in excess of ammonium hydroxide due to the formation of a deep blue complex, tetraamminecopper(II) ([Cu(NH3)4]2+), making it soluble.
Related Questions
Given below in column A is a schematic diagram of the electrolytic reduction of alumina. Identify the parts labelled as A, B and C with the correct options from the Column B.

Element 'X' forms an oxide with the formula X2O3 which is a solid with high melting point. 'X' would most likely be placed in the group of the Periodic Table as:
(a) Na
(b) Mg
(c) Al
(d) SiJustify your answer in the above question (1).
Copper sulphate solution is electrolysed using copper electrodes.
(a) Which electrode [cathode or anode] is the oxidizing electrode? Why?
(b) Write the equation for the reaction occurring at the above electrode.
X [2, 8, 7] and Y [2, 8, 2] are two elements. Using this information complete the following:
(a) …………… is the metallic element.
(b) Metal atoms tend to have a maximum of …………… electrons in the outermost shell.
(c) …………… is the reducing agent.