Chemistry
Assertion (A): An atom is the smallest part of matter which can take part in a chemical reaction.
Reason (R): Atoms of every element can exist independently.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — An atom is the smallest particle of an element and can take part in a chemical reaction. Hence, the assertion (A) is true.
Atoms may or may not exist independently.
For example:
Noble gas atoms like He, Ne can exist freely.
But atoms like H, O, and N do not exist alone under normal conditions; they exist as diatomic molecules (H2, O2, N2). Hence, reason (R) is false.
Related Questions
The figure given below shows the molecule of an element, where 'a' denotes the atom with atomic mass 32.
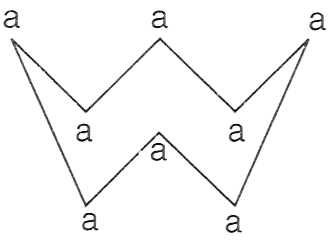
P — Element is tetratomic with molecular mass 128.
Q — Element is octatomic with molecular mass 256.
R — Element is crown-shaped with molecular mass 256.
Only P
Only Q
Only R
Both P and R
Assertion (A): The atomic mass of sodium is 23 amu.
Reason (R): An atom of sodium is 23 times heavier than an atom of carbon with mass 12 amu.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): All equations need to be balanced.
Reason (R): An unbalanced equation would imply that atoms have been created or destroyed.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): PCl3, is known as phosphorus trichloride while AlCl3, is aluminium chloride and not aluminium trichloride.
Reason (R) : Phosphorus shows variable valency. Aluminium does not show variable valency
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.