Physics
During the change of state of a substance from solid to liquid, heat is absorbed by a body, but its temperature does not rise. Which of the following statements explains the phenomenon?
- Only K.E of the molecules increases, but P.E remains the same.
- Only P.E of the molecules increases, but K.E remains the same.
- Both K.E and P.E of the molecules increase.
- There is no increase in the P.E and K.E of the molecules.
Answer
Only the P.E of the molecules increases, but the K.E remains the same.
Reason— When a substance changes from a solid to a liquid, the heat energy absorbed is used to overcome the intermolecular forces holding the solid particles together, thus increasing the potential energy (P.E.) of the molecules. However, the kinetic energy (K.E.), which relates to the vibrational motion of the molecules, does not change significantly during this phase change. The added energy goes entirely into breaking the bonds between the molecules, not into increasing their speed of vibration.
Related Questions
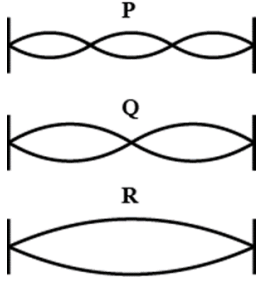
What is the ratio of wavelengths between P, Q and R?
- 3:2:1
- 1:2:3
- 6:3:2
- 2:3:6
Assertion (A) : When current is passed through a wire, a magnetic field is generated around the wire.
Reason(R) : Whenever there is a change of magnetic flux linked up with the coil, the induced current is generated in the coil.
- Both A and R are correct, and R is the correct explanation of A.
- Both A and R are correct, but R is not the correct explanation of A.
- A is correct but R is wrong.
- Both A and R are wrong.
Which of the following combinations correctly shows the charges on the particles?
+ 1.6 X 10-19 C - 1.6 X 10-19 C + 3.2 X 10-19 C (a) proton neutron alpha particle (b) alpha particle electron neutron (c) proton electron alpha particle (d) electron Proton alpha particle