Chemistry
Copper sulphate solution is electrolysed using copper electrodes.
(a) Which electrode [cathode or anode] is the oxidizing electrode? Why?
(b) Write the equation for the reaction occurring at the above electrode.
Answer
(a) In the electrolysis of copper sulphate solution using copper electrodes the anode is the oxidizing electrode. Copper atoms lose electrons at the anode and go into the solution as copper ions. Thus, the anode causes oxidation, making it the oxidizing electrode.
(b) Cu ⟶ Cu+2 + 2e-
Related Questions
Element 'X' forms an oxide with the formula X2O3 which is a solid with high melting point. 'X' would most likely be placed in the group of the Periodic Table as:
(a) Na
(b) Mg
(c) Al
(d) SiJustify your answer in the above question (1).
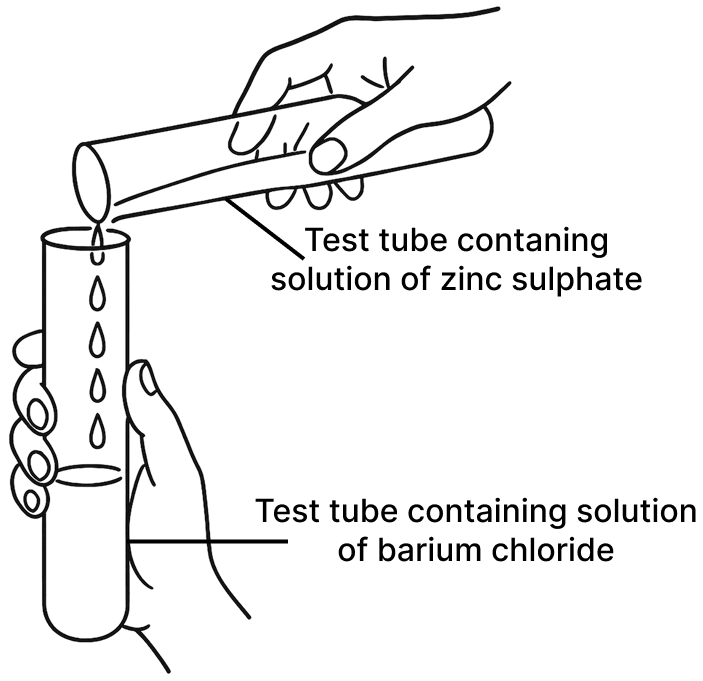
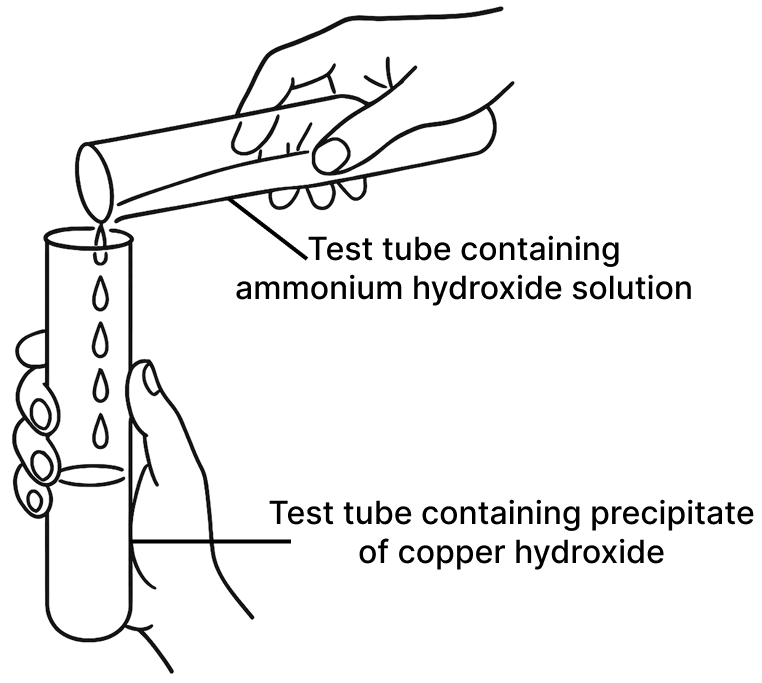
A student was asked to perform two experiments in the laboratory based on the instructions given:
Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
(a) Experiment 1
(b) Experiment 2
X [2, 8, 7] and Y [2, 8, 2] are two elements. Using this information complete the following:
(a) …………… is the metallic element.
(b) Metal atoms tend to have a maximum of …………… electrons in the outermost shell.
(c) …………… is the reducing agent.
One variety of household fuel is a mixture of propane (60%) and butane (40%). If 20 litres of this mixture is burnt, find the total volume of carbon dioxide added to the atmosphere. The combination reactions can be represented as:
C3H8 + 5O2 ⟶ 3CO2 + 4H2O
2C4H10 + 13O2 ⟶ 8CO2 + 10H2O