Physics
Which of the following equations are correctly balanced?
- Only D
- Only C
- Both A & B
- Both C & D
Radioactivity
9 Likes
Answer
Both C & D
Reason — A balanced nuclear equation must conserve:
- Mass number (top value) — total nucleons (protons + neutrons)
- Atomic number (bottom value) — total protons
Equation A
LHS: Mass number= q-1
Atomic number = p-5
RHS: mass number= (q-5 + 4) = q-1
Atomic number= (p-3 + 2) = p-1
so this equation is not balanced.
Equation B
LHS: Mass number= q-4
Atomic number = p-4
RHS: mass number= (q-4 + 0) = q-4
Atomic number= p-5 + (-1) = p-6
so this equation is not balanced.
Equation C
LHS: Mass number= q-1
Atomic number = p-5
RHS: mass number= (q-5 + 4) = q-1
Atomic number= (p-7 + 2) = p-5
so this equation is balanced.
Equation D
LHS: Mass number= q-4
Atomic number= p-7
RHS: Mass number= q-4 + 0 = q-4
Atomic number= p-5 + 2(-1) = p-7
so this equation is balanced.
Answered By
3 Likes
Related Questions
Assertion (A) : A switch will serve the purpose of making and breaking a circuit when it is connected to the neutral wire.
Reason(R) : Neutral wire is at zero potential.
- Both Assertion and Reason are true, and the Reason is the correct explanation of the Assertion
- Both Assertion and Reason are true, but Reason is not the correct explanation of the Assertion
- Assertion is true, but the Reason is false.
- Both Assertion & Reason are false.
The given rods will attract each other when:
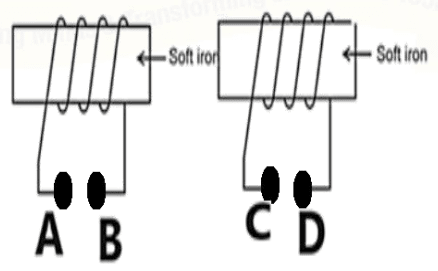
- A and C are connected to positive terminal of the battery.
- B and C are connected to the positive terminal of the battery.
- A and D are connected to the positive terminal of the battery.
- A and D are connected to the negative terminal of the battery.
The below picture shows three cylinders filled with water to a different extent. The tuning forks L, M and N in vibration produce louder sound when held above the mouth of the cylinders. Which combination is correct for hearing a louder sound of the tuning fork?
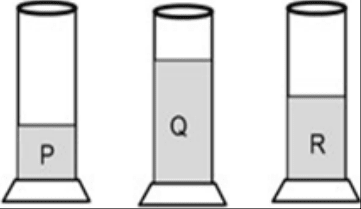

- L – R, M – Q, N – P
- L – P, M – Q, N – R
- L – R, M – P, N – Q
- L – Q, M – R, N – P
Three metals, A, B and C, are supplied with the same quantity of heat. Their masses are in the ratio 2 : 3 : 4. If they show the same rise in the temperature, then the ratio of their thermal capacities will be:
- 1:1:1
- 4:3:2
- 2:3:4
- 2:3:1