Chemistry
The gas evolved in the diagrammatic set up given below turns calcium hydroxide solution milky. The gas evolved is:
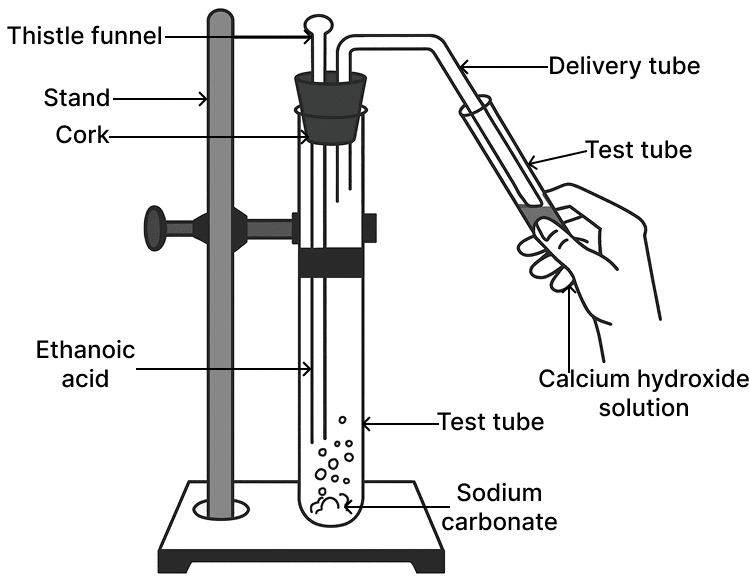
- CH4
- C2H6
- CO2
- SO2
Organic Chemistry
1 Like
Answer
CO2
Reason — The setup shows ethanoic acid reacting with sodium carbonate. This reaction produces carbon dioxide gas, which when passed through calcium hydroxide solution [lime water] turns it milky due to the formation of insoluble calcium carbonate:
Hence, the gas evolved is carbon dioxide (CO₂).
Answered By
2 Likes
Related Questions
A salt solution which gives a reddish-brown precipitate with NaOH and a white precipitate with BaCl2 solution is:
- CuSO4
- Ca(NO3)2
- Fe2(SO4)3
- FeCl3
An alkane with molecular mass 44 is:
- CH4
- C3H8
- C4H10
- C2H6
Which gas is evolved when ammonia gas is passed over buff yellow PbO?
- N2O
- NO
- N2
- NO2
Three different solutions X (sodium chloride solution), Y (acetic acid) and Z (sugar solution) were used for electrolysis by a student. When the circuit was completed, he noticed that the bulb glowed in the electrolytic cell containing:
- X & Y
- Y & Z
- Z & X
- X, Y & Z