Chemistry
Related Questions
Which is the most preferred metal for the laboratory preparation of hydrogen. Why is any other metal not used?
Describe the laboratory preparation of hydrogen with a labelled diagram? How are the impurities removed.
Look at the following figure and answer the questions that follow:

(a) Which gas is prepared by this method marked as A.
(b) Name this method of collection. Why is this method used?
(c) Why is nitric acid not used as a reactant in the above method?
(d) Conc. H2SO4 is a good drying agent. However, it is not used here. Why?
Identify the gas evolved in the figure shown below.
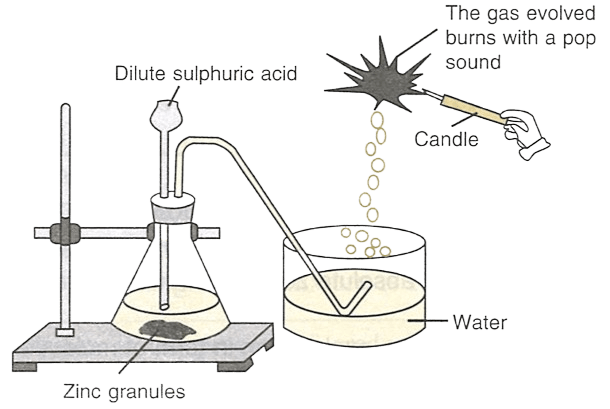
What would happen if the following changes are made:
(a) In place of zinc granules, same amount of zinc dust is taken in the test tube.
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) Sodium hydroxide is taken in place of dilute sulphuric acid and it is heated.