Physics
What mass of ice at 0°C added to 2.1 kg water, will cool it down from 75°C to 25°C? Given Specific heat capacity of water = 4.2 J g⁻¹ °C⁻¹, Specific latent heat of ice = 336 J g⁻¹.
Calorimetry
1 Like
Answer
Given,
For Water
Mass of water (mw) = 2.1 kg = 2100 g
Initial temperature (Ti) = 75°C
Final temperature (Tf) = 25°C
Specific heat capacity of water (cw) = 4.2 J g⁻¹ °C⁻¹
For ice
Initial temperature (Ti) = 0°C
Final temperature (Tf) = 25°C
Specific latent heat of fusion (Li) = 336 J g⁻¹
Let,
Mass of ice = (mi)
Now,
Heat lost by water = mwcw△t
= 2100 × 4.2 × (75 - 25)
= 2100 × 4.2 × 50 = 441000 J = 441 kJ
Heat gained by ice = miLi + micw△t
= mi × 336 + mi × 4.2 × (25-0)
= 336mi + mi × 4.2 × 25
= 336mi + 105mi = 441mi
By principle of calorimetry,
Heat lost by water = Heat gained by ice
⟹ 441000 = 441 mi
⟹ mi = = 1000 g = 1 kg
So, the mass of required ice is 1 kg.
Answered By
1 Like
Related Questions
A radioactive nucleus X emits an alpha particle followed by two beta particles to form nucleus Y.
(a) With respect to the element X, where would you position the element Y in the periodic table?
(b) What is the general name of the elements X and Y?
(c) If the atomic number of Y is 80 then what is the atomic number of X?
Observe the given circuit diagram and answer the questions that follow :
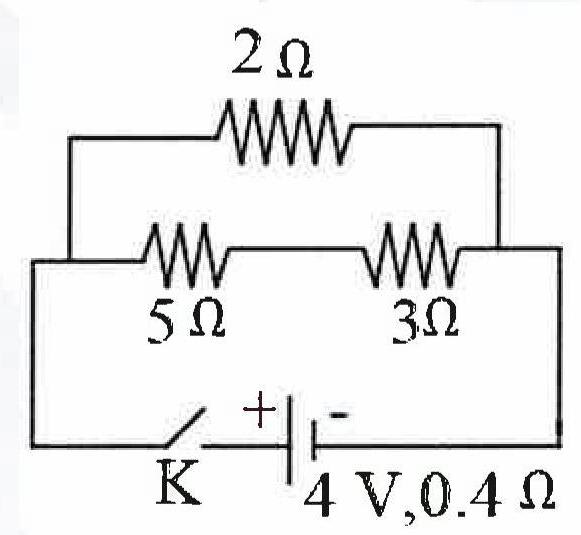
(a) Calculate the resistance of the circuit when the key K completes the circuit.
(b) Calculate the current through 3 Ω resistance when the circuit is complete.
The diagram below shows a cooling curve for a substance :
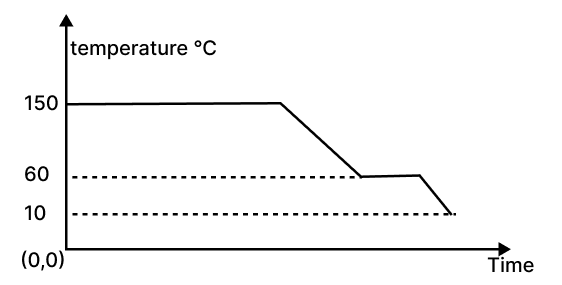
(a) State the temperatures at which the substance condenses.
(b) The temperature range in which the substance is in liquid state.
(c) Why do we prefer ice to ice-cold water for cooling a drink?
The diagram below shows a cardboard on which iron filings are kept. A wire bent in the form of a loop is seen passing through the cardboard. When current flows through it the iron filings arrange themselves as shown below with the direction of magnetic field.
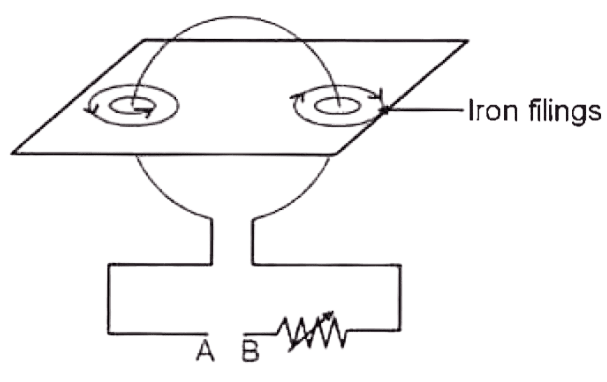
(a) State the polarities of the battery at A and B.
(b) State the effect on the magnetic field if an iron rod is held along the axis of the coil.
(c) State one way to :
- Change the polarity of the coil.
- Decrease the strength of the magnetic field around the coil.