Physics
Observe the given circuit diagram and answer the questions that follow :
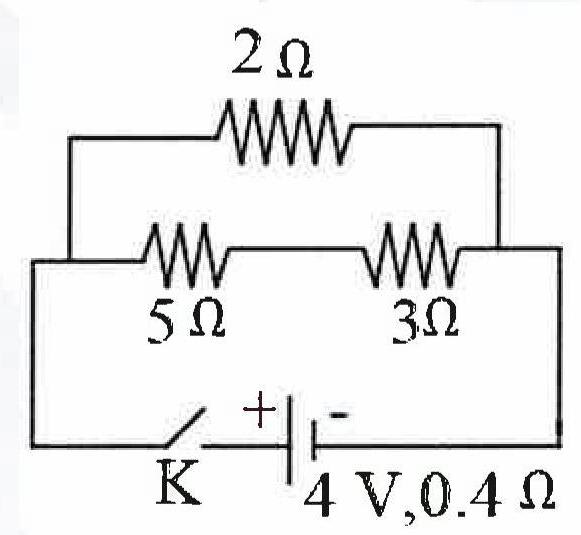
(a) Calculate the resistance of the circuit when the key K completes the circuit.
(b) Calculate the current through 3 Ω resistance when the circuit is complete.
Radioactivity
2 Likes
Answer
(a) 5Ω and 3Ω are in series so their equivalent resistance (Rs) = 5 + 3 = 8Ω
Now 2Ω and Rs are in parallel.
From circuit diagram,
Internal Resistance = 0.4 Ω
Total resistance of circuit = 1.6 + 0.4 = 2 Ω
(b) Current drawn from the battery :
Now, the current I divides in 2 parts.
Let current across 2Ω be I1 and across the series combination of 5Ω and 3Ω be I2.
I = I1 + I2 = 2 A …………..(1)
Terminal Voltage of cell = V = IRp = 2 x 1.6 = 3.2 V
∴ P.d. across 2 Ω resistor = 3.2 V
I1 = = 1.6 A
Substituting value of I1 in Eq 1,
2 = 1.6 + I2
⇒ I2 = 2 - 1.6 = 0.4 A
Hence, current across 3Ω = 0.4 A
Answered By
1 Like
Related Questions
The circuit depicted in the figure is employed for studying Ohm's Law. Instead of using a standard resistor, a student opts for a glass tube filled with mercury (tube 1), connected to the circuit through two electrodes E1 & E2. He records the readings of the ammeter and voltmeter, thereby calculates the resistance. The student repeats the experiment by substituting tube 1 with tube 2, where the same amount of mercury fills the tube 2.
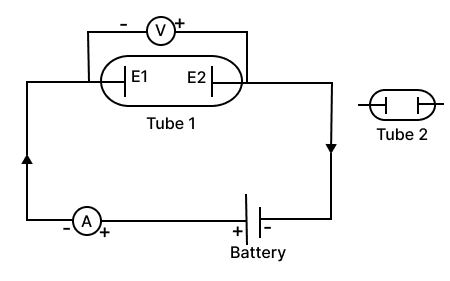
Neglecting internal resistance of the cell use (> or < or =) to compare
(a) the resistance in both the cases.
(b) the voltmeter readings in both the cases.
(c) the specific resistance in both the cases.
A radioactive nucleus X emits an alpha particle followed by two beta particles to form nucleus Y.
(a) With respect to the element X, where would you position the element Y in the periodic table?
(b) What is the general name of the elements X and Y?
(c) If the atomic number of Y is 80 then what is the atomic number of X?
What mass of ice at 0°C added to 2.1 kg water, will cool it down from 75°C to 25°C? Given Specific heat capacity of water = 4.2 J g⁻¹ °C⁻¹, Specific latent heat of ice = 336 J g⁻¹.
The diagram below shows a cooling curve for a substance :
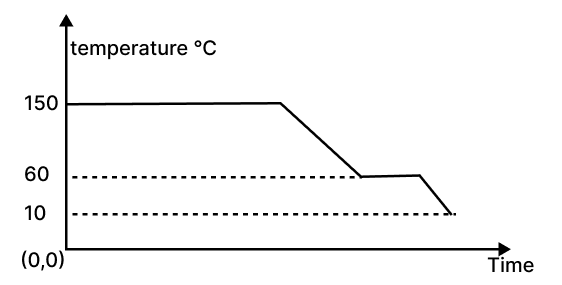
(a) State the temperatures at which the substance condenses.
(b) The temperature range in which the substance is in liquid state.
(c) Why do we prefer ice to ice-cold water for cooling a drink?